Cement kiln harmless pharmaceutical factory waste residue co-processing method
A technology of co-processing and cement kiln, applied in the direction of solid waste removal, etc., can solve the problems of secondary pollution, complicated disposal of waste drug residues, and high processing cost, and achieve the effect of simplifying the disposal process, shortening the disposal period, and reducing environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
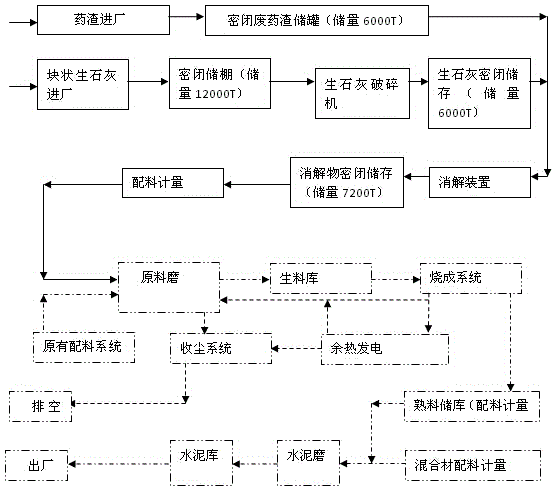
[0028] A method for the harmless co-processing of waste drug residues in pharmaceutical factories in cement kilns, the flow chart is shown in figure 1 shown, including the following steps:
[0029] Fill penicillin mycelia waste residue with a water content of 95wt% (that is, a solid content of 5wt%) into a closed waste drug residue storage tank (1000t in storage capacity), a total of six sealed waste drug residue storage tanks are equipped with explosion-proof and gas collection devices;
[0030] Put the lumpy quicklime into the storage shed (12000t in reserve); put the lumpy quicklime in the storage shed into the crusher for crushing, and send it to the steel plate storage shed through the conveying equipment (6000t in storage);
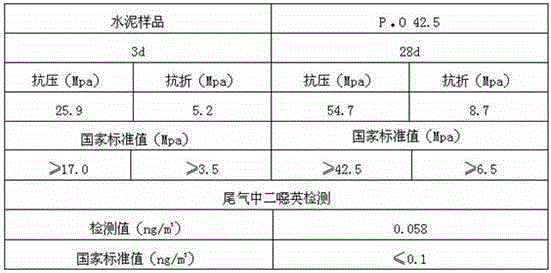
[0031] ③ Combine the crushed quicklime with the step The penicillin mycelia waste residue in the medium is added to the digestion device for digestion in a ratio of 1:2.8 by weight to obtain a digested product. The digestion time is 5min, and...
Embodiment 2
[0039] A method for the harmless co-processing of waste drug residues in pharmaceutical factories in cement kilns, the flow chart is shown in figure 1 shown, including the following steps:
[0040] Inject the oxytetracycline mycelia waste residue with a moisture content of 90wt% (i.e. a solid content of 10wt%) into the sealed waste drug residue storage tank (1000t storage capacity), a total of six sealed waste drug residue storage tanks are equipped with explosion-proof and gas collection devices;
[0041] Put the lumpy quicklime into the storage shed (12000t in reserve); put the lumpy quicklime in the storage shed into the crusher for crushing, and send it to the steel plate storage shed through the conveying equipment (6000t in storage);
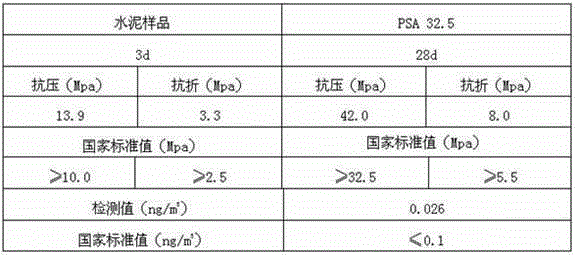
[0042] ③ Combine the crushed quicklime with the step The oxytetracycline mycelia waste residue in the solution was added to the digestion device in a weight ratio of 1: 2 for digestion to obtain a digested product. The digestion tim...
Embodiment 3
[0050] A method for the harmless co-processing of waste drug residues in pharmaceutical factories in cement kilns, the flow chart is shown in figure 1 shown, including the following steps:
[0051] Streptomycin mycelia waste residue with a moisture content of 75wt% (that is, a solid content of 25wt%) is injected into a closed waste drug residue storage tank (1000 tons of storage capacity), a total of six sealed waste drug residue storage tanks are equipped with explosion-proof and gas collection devices;
[0052] Put the lumpy quicklime into the storage shed (12000t in reserve); put the lumpy quicklime in the storage shed into the crusher for crushing, and send it to the steel plate storage shed through the conveying equipment (6000t in storage);
[0053] ③ Combine the crushed quicklime with the step The streptomycin mycelia waste residue in the solution was added to the digestion device for digestion in a ratio of 1: 1.2 by weight to obtain a digested product. The dige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com