Method for separating cyclohexanol and cyclohexanone from ka oil
A technology for cyclohexanone and cyclohexanol, which is applied in the field of separation of cyclohexanol and cyclohexanone, can solve the problems of high cost, high energy consumption, complicated operation, etc., achieve reduced energy consumption and production cost, high separation efficiency, simplified The effect of the process step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the stirred reactor equipped with KA oil, slowly add 55% hydrogen peroxide to carry out the stirring reaction, keep the temperature of the stirred reactor at 15°C, the reaction will continue to precipitate cyclohexanone peroxide precipitation, until the reaction proceeds to no precipitation, stop reaction, filtration and separation to obtain cyclohexanone peroxide, and the filtrate obtained by filtration and separation was left to cool in a cold water bath at 5°C, and the upper layer solid was collected to obtain cyclohexanol; the separated cyclohexanone peroxide was placed in a large amount of water and heated to 60 ℃ for hydrolysis reaction, after the completion of the hydrolysis reaction, cooling, collecting the upper layer of oily liquid, that is, cyclohexanone. The cyclohexanone and cyclohexanol products collected are carried out chromatographic analysis, and cyclohexanol purity is 97.5%, and cyclohexanone purity is 98%; And calculate according to raw material co...
Embodiment 2
[0026]In the stirred reactor equipped with KA oil, slowly add 35% hydrogen peroxide to carry out the stirring reaction, keep the temperature of the stirred reactor at 30°C, the reaction will continue to precipitate cyclohexanone peroxide precipitation, until the reaction proceeds to no precipitation, stop reaction, filtration and separation to obtain cyclohexanone peroxide, and the filtrate obtained by filtration and separation was left to cool in an ice-water bath, and the upper layer solid was collected to obtain cyclohexanol; the obtained cyclohexanone peroxide was placed in a large amount of water and heated to 70°C for Hydrolysis reaction, after the completion of the hydrolysis reaction, cool down, collect the oily liquid in the upper layer, and obtain cyclohexanone. The cyclohexanone and cyclohexanol products collected are carried out chromatographic analysis, and cyclohexanol purity is 96.1%, and cyclohexanone purity is 97%; And calculate according to raw material compos...
Embodiment 3
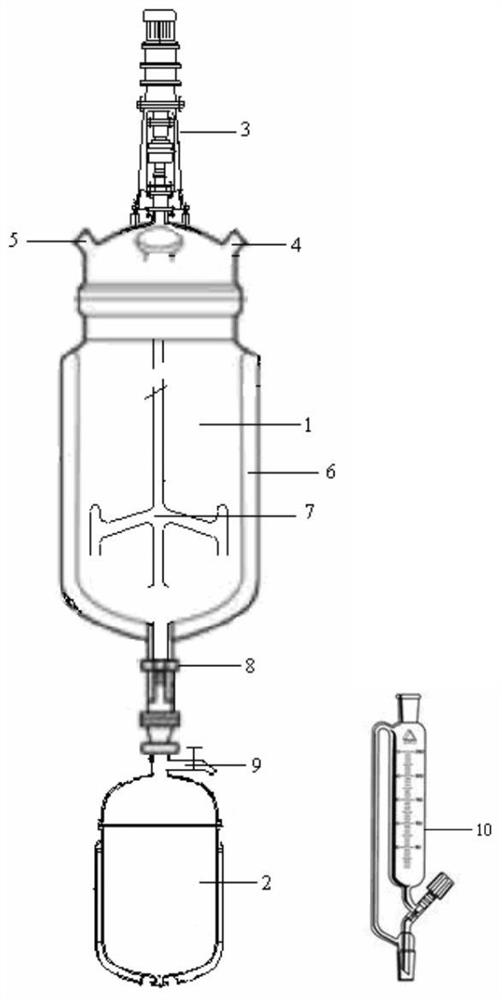
[0028] by figure 1 The device realizes the separation of KA oil. First open the valve between the settling tower and the stirring reaction tower to connect the two, inject KA oil from the KA oil inlet until the KA oil covers the stirring bar at the bottom of the agitator, add 45% hydrogen peroxide from the hydrogen peroxide inlet, and control The temperature in the stirred reaction tower is 40°C. Under the stirring condition, the hydrogen peroxide and the cyclohexanone in the KA oil fully contact and react in the stirred reaction tower. It settles into the settling tower under its own gravity, and the KA oil in the settling tower is pushed up by the settled cyclohexanone peroxide and rises into the stirring reaction tower, and the reaction continues until the settling tower is filled with cyclohexanone peroxide. When oxidation occurs, close the valve between the settling tower and the stirring reaction tower, open the discharge branch pipe to recover a small amount of reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com