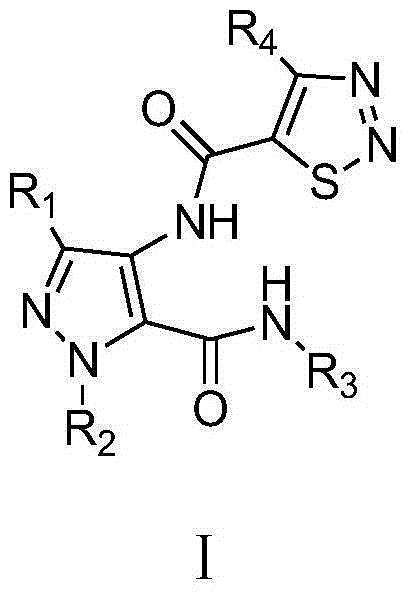
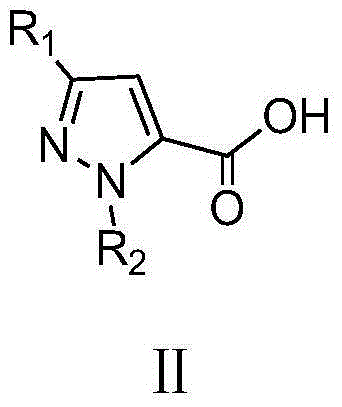
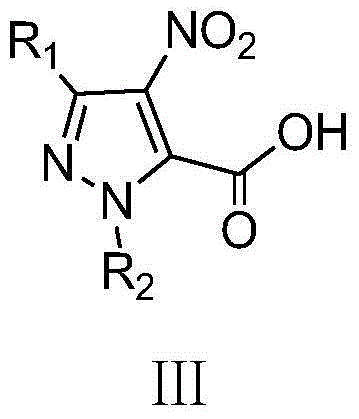
Pyrazol bi-amide compounds containing 1,2,3-thiadiazole and synthetic method and application thereof
A technology of pyrazole bisamides and thiadiazoles, applied in the field of pyrazole bisamides and their synthesis, can solve the problems of weak lipophilicity and strong hydrophilicity, and achieve good armyworm killing activity and room for modification and transformation The effect of large size and reasonable and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1N-(3-ethyl-5-(methylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide
[0038] (1) 3-Ethyl-4-nitro-1H-pyrazole-5-carboxylic acid
[0039] Add 7.5mL (183.78mmol) fuming nitric acid into a 50mL three-necked flask, slowly add 8.4mL (170.57mmol) of fuming sulfuric acid dropwise under ice bath, control the system temperature below 5°C during the dropping process, add 3- Ethyl-1H-pyrazole-5-carboxylic acid 7.0g (50mmol), reacted at 60°C for 18h, cooled to room temperature, poured the reaction solution into 100g of ice, white precipitate appeared, suction filtered, and dried to obtain 6.64g of white solid, Yield: 71.78%, m.p.150~152℃;
[0040] (2) N-methyl-3-ethyl-4-nitro-1H-pyrazole-5-carboxamide
[0041] Add 10g (54mmol) of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid and 51mL (702mmol) of thionyl chloride into a 100mL three-necked flask, heat to reflux for 4h, after the reaction is complete, remove the unreacted mixture under reduced pressure ...
Embodiment 2
[0050] Example 2N-(3-ethyl-5-(ethylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide
[0051] (1) The preparation of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid refers to Example 1;
[0052] (2) N,3-diethyl-4-nitro-1H-pyrazole-5-carboxamide Preparation Reference Example 1: white solid, yield: 41.01%, m.p.150-152°C. 1 HNMR (CDCl 3 ,ppm):1.28~1.35(m,6H),2.99~3.07(q,J=7.4Hz,2H),3.52~3.61(m,2H),9.04(s,1H),12.20(s,1H); MS:210.8[M-H] - ;
[0053] (3) The preparation of N,3-diethyl-4-amino-1H-pyrazole-5-carboxamide refers to Example 1;
[0054] (4) The preparation of 4-methyl-1,2,3-thiadiazole-5-carbonyl chloride refers to Example 1;
[0055] (5) N-(3-ethyl-5-(ethylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide The preparation reference example 1: white solid, yield: 73.78%, m.p.196~197 ℃; 1 HNMR (CH 3 OH-d 4 , ppm): 1.18~1.23(t, J=7.2Hz, 3H), 1.24~1.29(t, J=7.6Hz, 3H), 2.71~2.78(q, J=7.6Hz, 2H), 2.94(s, 3H), 3.39~3.42(q, J=7.2Hz, ...
Embodiment 3
[0056] Example 3N-(3-ethyl-5-(n-propylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide
[0057] (1) The preparation of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid refers to Example 1;
[0058] (2) Reference Example 1 for the preparation of 3-ethyl-N-n-propyl-4-nitro-1H-pyrazole-5-carboxamide: white solid, yield: 73.00%, m.p.132~134°C; 1 HNMR (DMSO, ppm): 0.87~0.92(t, J=7.4Hz, 3H), 1.20~1.25(t, J=7.5Hz, 3H), 1.47~1.54(m, 2H), 2.86~2.94(q, J=7.5Hz, 2H), 3.19(m, 2H), 8.50(s, 1H); MS: 227.1[M+H] + ;
[0059] (3) The preparation of 3-ethyl-N-n-propyl-4-amino-1H-pyrazole-5-carboxamide refers to Example 1;
[0060] (4) The preparation of 4-methyl-1,2,3-thiadiazole-5-carbonyl chloride refers to Example 1;
[0061] (5) N-(3-ethyl-5-(n-propylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-methanol The preparation of amides refers to Example 1: white solid, yield: 93.83%, m.p.186~187°C; 1 HNMR (CH 3 OH-d 4 , ppm): 0.93~0.98(t, J=7.4Hz, 3H), 1.23~1.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com