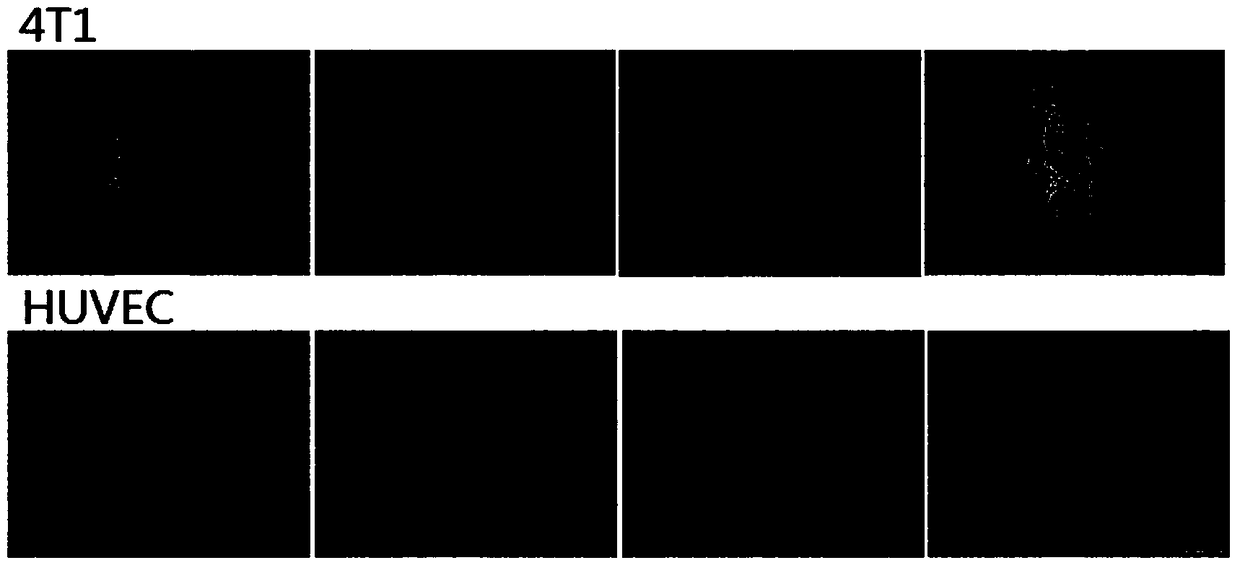
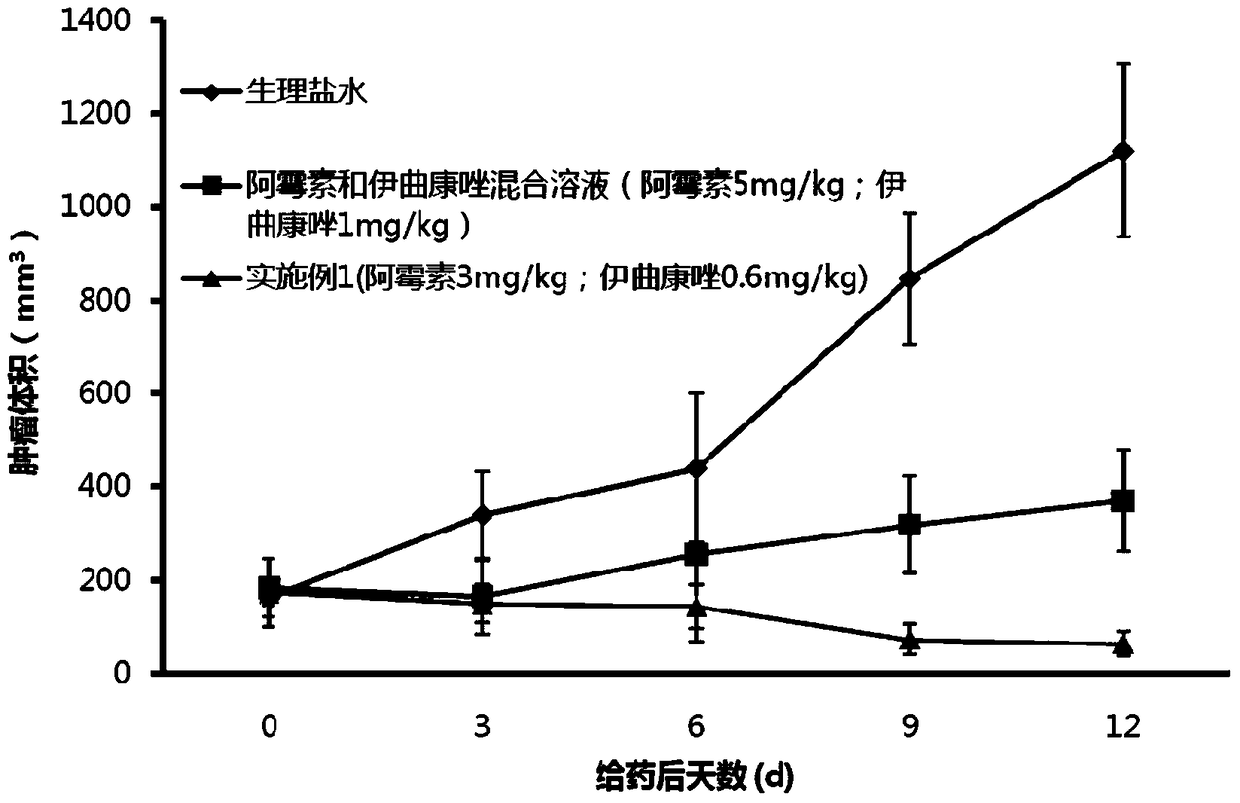
Doxorubicin and itraconazole co-loaded nanoliposomes and preparation method thereof
An itraconazole nano- and nano-liposome technology, which is applied in the directions of liposome delivery, pharmaceutical formulations, and medical preparations containing active ingredients, can solve the lack of evaluation results of in vivo tumor-bearing animals, insufficient treatment results Comprehensive evaluation of drug efficacy and toxic side effects, and normal tissue toxic side effects, etc., to achieve the effect of inhibiting vascular endothelial cell proliferation, improving tumor treatment effect, and inhibiting tumor angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The processing steps of the present embodiment are as follows:
[0037] (1) Preparation of itraconazole nanoliposomes
[0038] Take by weighing itraconazole 10g (2 mass parts), soybean lecithin (soybean phosphatidylcholine purity > 76%) 540g (108 mass parts), cholesterol 400g (80 mass parts), each raw material that weighs is dissolved together Form solution in the combination solvent that methanol and dichloromethane combine gained with volume ratio 1:2, the consumption of described combination solvent is limited to 1mg / L with soybean lecithin concentration in solution, remove solvent and obtain lipid film, the gained lipid film Soluble in 250mmol / L (NH 4 ) 2 SO 4 A suspension is formed in the solution, (NH 4 ) 2 SO 4 The amount of solution used is limited to the concentration of soybean lecithin in the suspension formed at 0.1 mg / mL. Ultrasonic the obtained suspension in a water bath at 30°C for 15 minutes, and ultrasonically with a probe at room temperature for ...
Embodiment 2
[0043] The processing steps of the present embodiment are as follows:
[0044] (1) Preparation of itraconazole nanoliposomes
[0045] Weigh itraconazole 100g (20 mass parts), phosphatidylglycerol 280g (56 mass parts), hydrogenated soybean phosphatidylcholine 420g (84 mass parts), cholesterol 150g (30 mass parts), polyethylene glycol modified Cholesterol 45g (9 mass parts), each raw material that weighs is dissolved in methanol and dichloromethane to form a solution in the combined solvent of the combination gained of volume ratio 1:2, the consumption of described combined solvent is based on the phospholipid (phospholipid) in the solution The total mass of acylglycerol and hydrogenated soybean phosphatidylcholine) concentration is limited to 200mg / L, and the solvent is removed to obtain a lipid film, and the gained lipid film is dissolved in 400mmol / L (NH 4 ) 2 SO 4 A suspension is formed in the solution, (NH 4 ) 2 SO 4 The amount of solution used is limited to the conce...
Embodiment 3
[0050] The processing steps of the present embodiment are as follows:
[0051] (1) Preparation of itraconazole nanoliposomes
[0052] Weigh itraconazole 20g (4 mass parts), phosphatidylserine 180g (36 mass parts), hydrogenated egg yolk phosphatidylcholine 400g (80 mass parts), cholesterol 100g (20 mass parts), polyethylene glycol modified Distearoylphosphatidylglycerol 100g (20 parts by mass), each weighed raw material is dissolved in the combined solvent obtained by combining methanol and chloroform with a volume ratio of 1:2 to form a solution, the amount of the combined solvent With the concentration of phospholipids (the total mass of phosphatidylserine and hydrogenated egg yolk phosphatidylcholine) in the solution being limited to 50 mg / L, remove the solvent to obtain a lipid film, and dissolve the gained lipid film in 300 mmol / L (NH 4 ) 2 SO 4 A suspension is formed in the solution, (NH 4 ) 2 SO 4 The amount of solution used is limited to the concentration of phosp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com