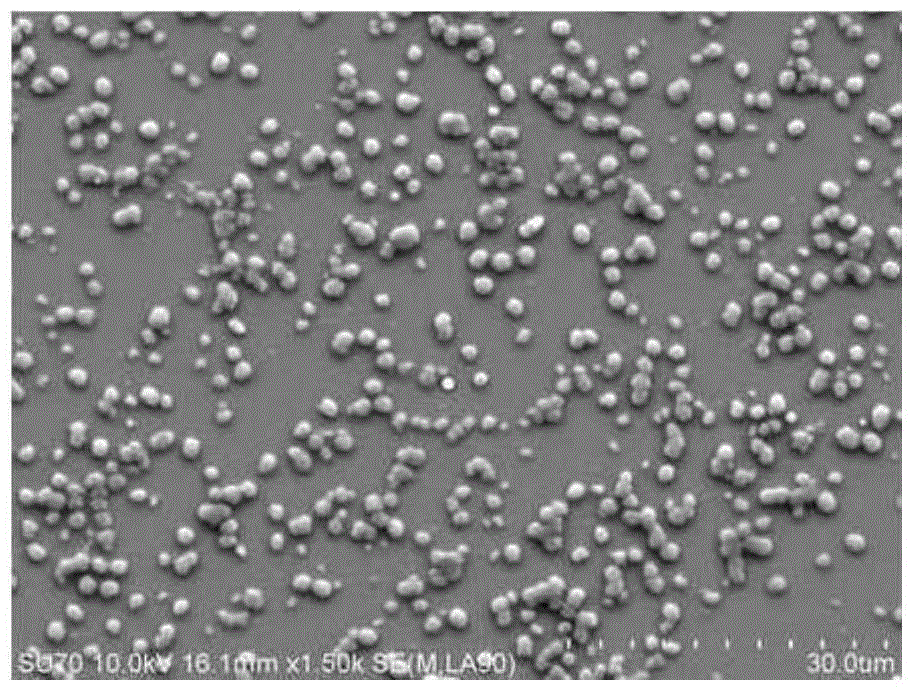
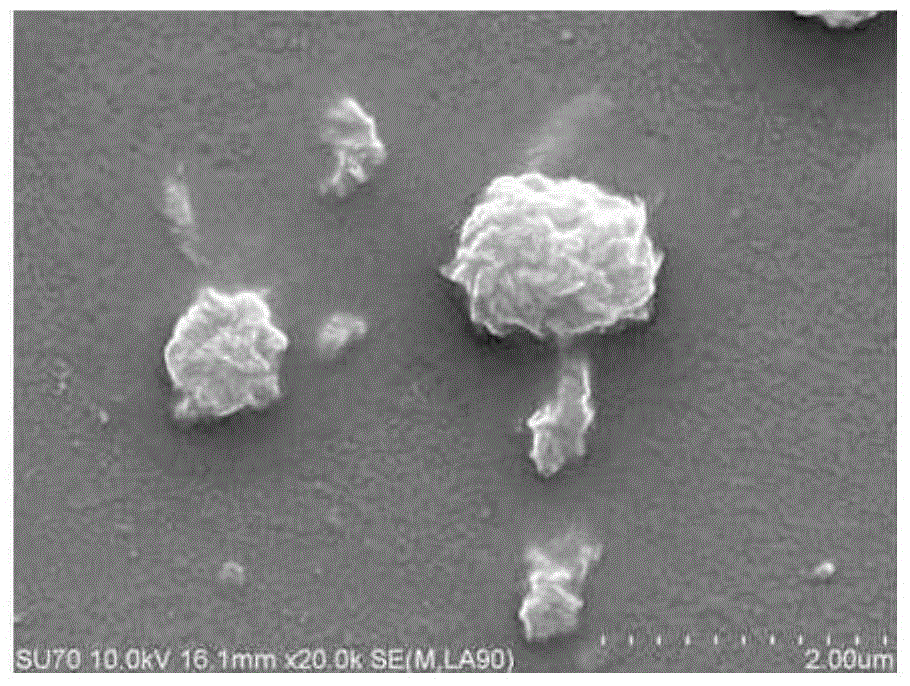
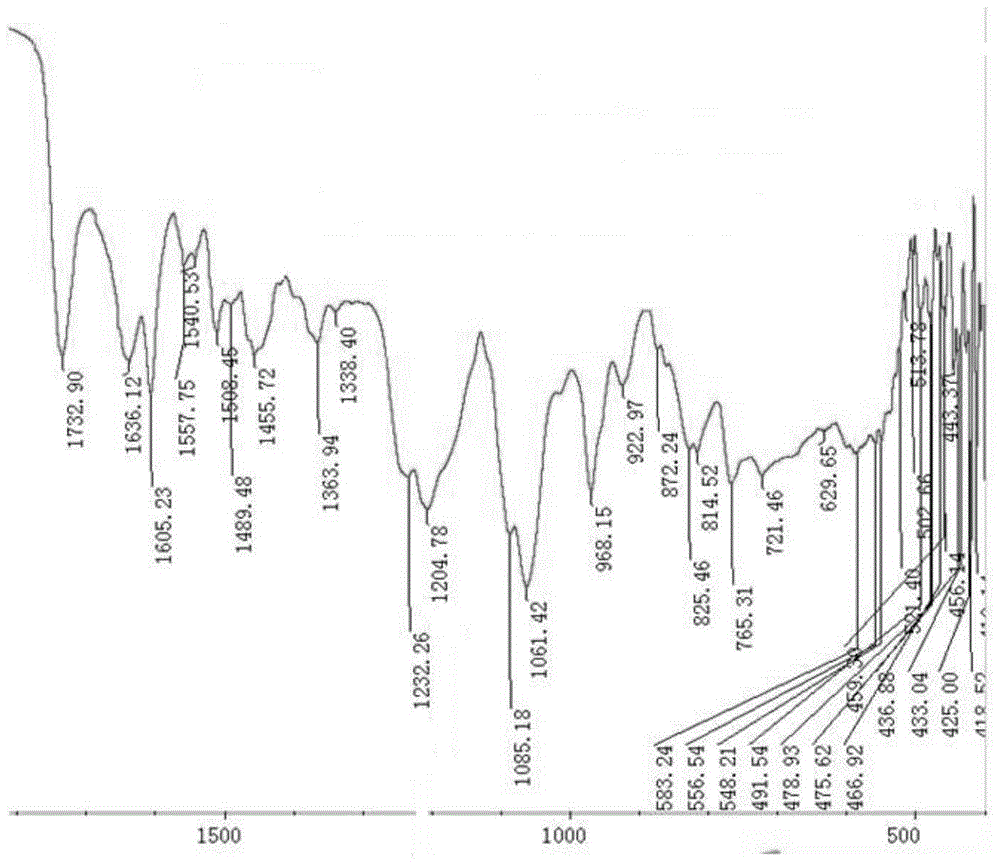
Preparation methods of methotrexate and lecithin compound, nano-particles of methotrexate and lecithin compound as well as targeted sustained release preparation of methotrexate and lecithin compound
A technology of methotrexate phospholipid and aminopterate phospholipid, which is applied in the direction of anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve the problems of high cost, long cycle for new drug application, complicated preparation process, etc., and achieve good stability, Low toxicity therapeutic effect, good tumor targeting and sustained release properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In the preparation method of the methotrexate phospholipid complex in this embodiment, methotrexate and lecithin are dissolved in the organic solvent A, and the mixing reaction is carried out at 20° C. The mixing reaction time is 12 hours. The organic solvent A in the resulting mixed solution is removed by vacuum rotary evaporation, and then the dried product after removing the organic solvent A is purified and further dried by using organic solvent B to obtain the methotrexate phospholipid complex, the organic solvent A It is one or more of tetrahydrofuran, dimethylformamide and acetone, and the organic solvent B is one or two mixtures of dichloromethane and chloroform. The mixing reaction is carried out in a water bath mode. The obtained methotrexate phospholipid complex was sequentially purified and dried. The molar ratio of methotrexate to lecithin is 1:1. The lecithin is egg yolk lecithin or soybean lecithin.
[0048] In the method for preparing methotrexate pho...
Embodiment 2
[0051] In the preparation method of the methotrexate phospholipid complex in this embodiment, methotrexate and lecithin are dissolved in the organic solvent A, and the mixing reaction is carried out at 40° C. The mixing reaction time is 12 hours. The organic solvent A in the mixed solution is removed by vacuum rotary evaporation, and then the dried product after removing the organic solvent A is purified and further dried by using the organic solvent B to obtain the methotrexate phospholipid complex, the organic solvent A is one or more of tetrahydrofuran, dimethylformamide and acetone, and the organic solvent B is one or more of chloroform and dichloromethane. The mixing reaction is carried out in a water bath mode. The obtained methotrexate phospholipid complex was sequentially purified and dried. The molar ratio of methotrexate to lecithin is 1:4. The lecithin is egg yolk lecithin or soybean lecithin.
[0052] In the method for preparing methotrexate phospholipid complex...
Embodiment 3
[0055] In the preparation method of the methotrexate phospholipid complex in this embodiment, methotrexate and lecithin are dissolved in the organic solvent A, and the mixing reaction is carried out at 30° C. The mixing reaction time is 24 hours. The organic solvent A in the resulting mixed solution is removed by vacuum rotary evaporation, and then the dried product after removing the organic solvent A is purified and dried by using organic solvent B to obtain the methotrexate phospholipid complex, the organic solvent A It is one or more of tetrahydrofuran, dimethylformamide and acetone, and the organic solvent B is one or more of chloroform and dichloromethane. The mixing reaction is carried out in a water bath mode. The obtained methotrexate phospholipid complex was sequentially purified and dried. The molar ratio of methotrexate to lecithin is 1:2. The lecithin is egg yolk lecithin or soybean lecithin.
[0056] In the method for preparing methotrexate phospholipid comple...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com