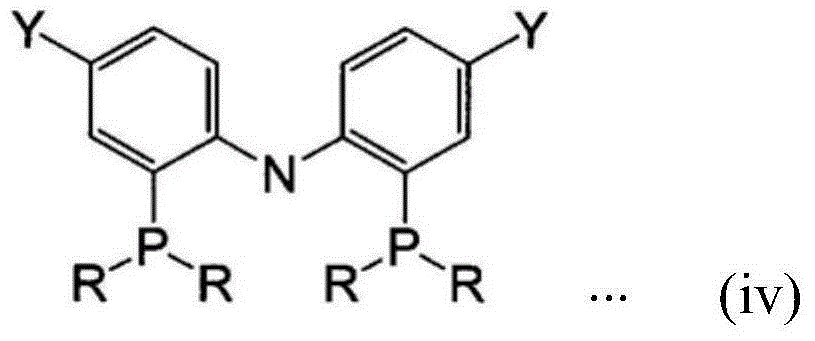
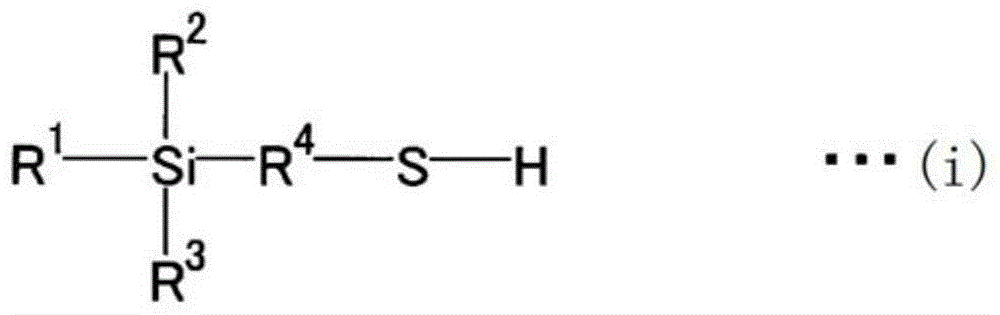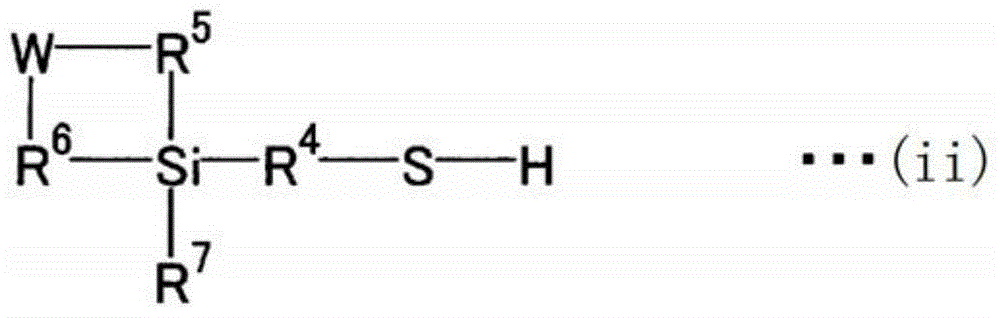Isoprene polymerization catalyst composition, method for producing synthetic polyisoprene, and synthetic polyisoprene
A polymerization catalyst, polyisoprene technology, applied in the field of polyisoprene synthesis, can solve problems such as low durability and difficulty in efficiently producing high molecular weight polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] (Example 1: Method for producing synthetic polyisoprene A)
[0140] In a glove box under a nitrogen atmosphere, 7.9 μmol tris(tert-butoxy)gadolinium (Gd(OtBu) 3 ) (component (a)), 1.19 mmol of triisobutylaluminum (component (c)) and 5.0 g of toluene were charged into a 1L pressure-resistant glass reactor. After aging for 30 minutes, 7.9 μmol of triphenylcarbon tetrakis (pentafluorophenyl) borate (Ph 3 CB(C 6 f 5 ) 4 ) (component (b)) and aged for 15 minutes. Then the reactor was taken out from the glove box, 235.0 g of cyclohexane and 70 g of isoprene were added, and a polymerization reaction was performed at 25° C. for 15 hours. After the polymerization, 1 mL of an isopropanol solution containing 5% by mass of 2,2'-methylene-bis(4-ethyl-6-tert-butylphenol) (NS-5) was added to terminate the reaction. Further, the polymer was isolated using a large amount of methanol and vacuum-dried at 70° C., whereby synthetic polyisoprene A was obtained. The yield of the result...
Embodiment 2
[0141] (Example 2: Manufacturing method of synthetic polyisoprene B)
[0142] The same method as in Example 1 was used except that the polymerization was performed at 50° C. for 2 hours. As a result, synthetic polyisoprene B was obtained in a yield of 68 g.
Embodiment 3
[0143] (Example 3: Manufacturing method of synthetic polyisoprene C)
[0144] In a glove box under a nitrogen atmosphere, 7.9 μmol tris(tert-butoxy)gadolinium (Gd(OtBu) 3 ) (component (a)), 7.9 μmol 2-ethyl-1-hexanol (component (d)), 1.19 mmol triisobutylaluminum (component (c)) and 5.0 g toluene into 1L pressure-resistant glass reactor. After aging for 30 minutes, 7.9 μmol of triphenylcarbon tetrakis (pentafluorophenyl) borate (Ph 3 CB(C 6 f 5 ) 4 ) (component (b)) and aged for 15 minutes. Then, the reactor was taken out from the glove box, 235.0 g of cyclohexane and 70 g of isoprene were added, and a polymerization reaction was performed at 50° C. for 2 hours. After the polymerization, 1 mL of an isopropanol solution containing 5% by mass of 2,2'-methylene-bis(4-ethyl-6-tert-butylphenol) (NS-5) was added to terminate the reaction. Further, the polymer was separated using a large amount of methanol and vacuum-dried at 70° C., whereby synthetic polyisoprene C was obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com