One-pot preparation of 2-bromo-9,9-diphenylfluorene
A technology of diphenylfluorene and phenyl, which is applied in the field of organic synthesis, can solve the problems of high cost of trifluoromethanesulfonic acid, influence on reaction yield, and multiple systems, and achieve simplified post-treatment process, reliable process, and reduced side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
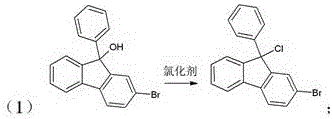
[0025] One-pot preparation of 2-bromo-9,9-diphenylfluorene, including the following steps:
[0026] (1) ;
[0027] (2) .
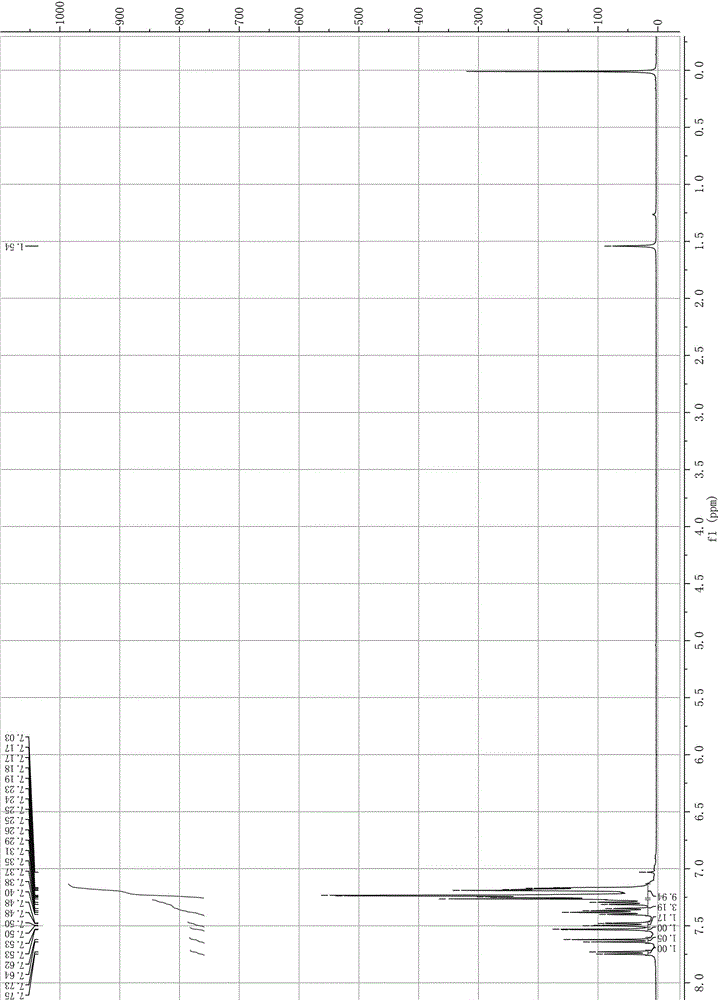
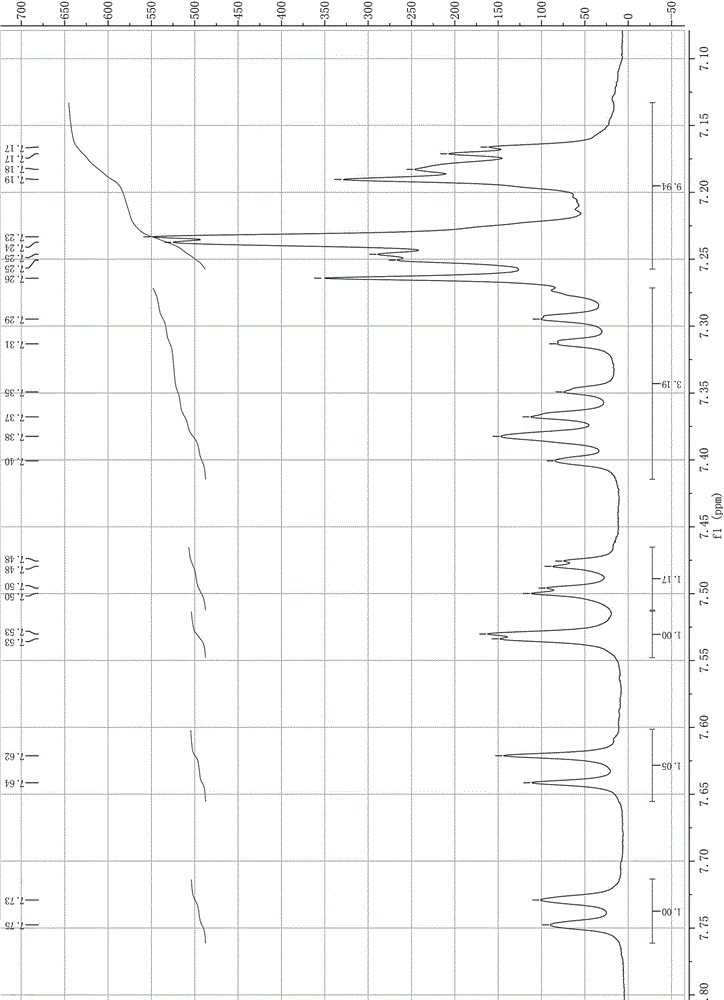
[0028] The specific operation of step (1) is: a) first mix 23 mmol thionyl chloride with 10 mmol 2-bromo-9-phenyl-fluoren-9-ol; b) then take 35 mmol thionyl chloride and heat to 60°C , and then dropwise add the mixed solution of step a) within 0.5h; c) chlorination reaction ends after heating up to reflux for 3h;
[0029] The specific operation of step (2) is: cool down the system after the chlorination reaction, add 8.5mmol of copper benzenesulfonate, then raise the temperature to 40°C, add 15mmol of benzene dropwise within 0.5h, and continue to heat up to 55°C for 5h , and finally filtered to remove copper benzenesulfonate, and the filtrate was concentrated under reduced pressure to obtain 2-bromo-9,9-diphenylfluorene with a yield of 95.7%.
Embodiment 2
[0031] In the present embodiment, the addition of copper benzenesulfonate is replaced by copper p-toluenesulfonate. Others are the same as in Example 1, and the final yield is 97.5%.
Embodiment 3
[0033] One-pot method for preparing 2-bromo-9,9-diphenylfluorene, the reaction formula is the same as in Example 1.
[0034] Include the following steps:
[0035] The specific operation of step (1) is: a) first mix 35 mmol phosphorus trichloride with 10 mmol 2-bromo-9-phenyl-fluoren-9-ol; b) then take 53 mmol phosphorus trichloride and heat to 70°C , and then dropwise add the mixed solution of step a) within 1 hour; c) the chlorination reaction ends after heating up and refluxing for 5 hours;
[0036] The specific operation of step (2) is: cool down the system after the chlorination reaction, add 11 mmol of copper p-toluenesulfonate, then raise the temperature to 48°C, add 22mmol of benzene dropwise within 0.5h, and continue to heat up to 60°C for reaction After 7 hours, the copper p-toluenesulfonate was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain 2-bromo-9,9-diphenylfluorene with a yield of 98.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com