Polypeptide drug preparation and preparation method thereof
A technology for pharmaceutical preparations and analogs, applied in the field of GLP-1 analog pharmaceutical preparations, can solve the problems of unachievable drug storage, reduced drug purity, and drug production, and achieve the effects of reducing drug risk, reducing toxic side effects, and reducing degradation.
Active Publication Date: 2015-12-09
QILU PHARMA
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, in the transportation and storage environment, the preservation of drugs cannot meet the above requirements. Drugs are easily affected by the environment, such as adsorption, degradation, aggregation, oxidation and other changes that affect drug stability, resulting in drug impurities and reducing drug purity.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
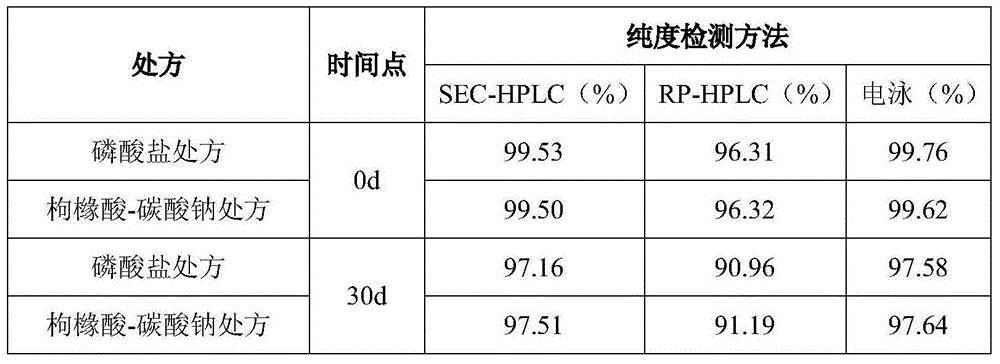
Effect test
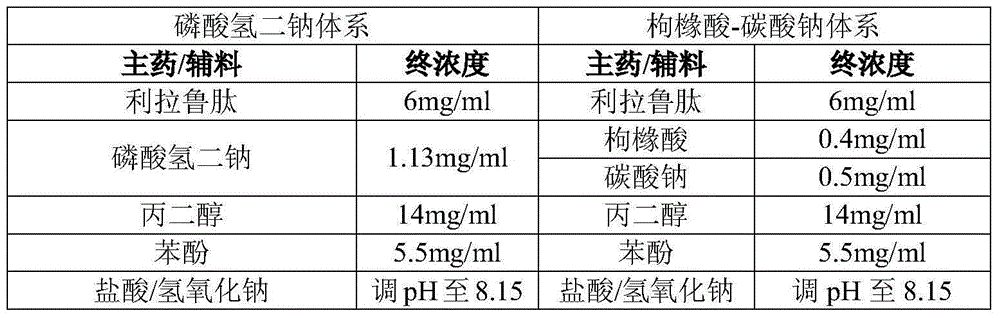
Embodiment 1
[0040] Main drug / excipient
Embodiment 2
[0042] Main drug / excipient
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
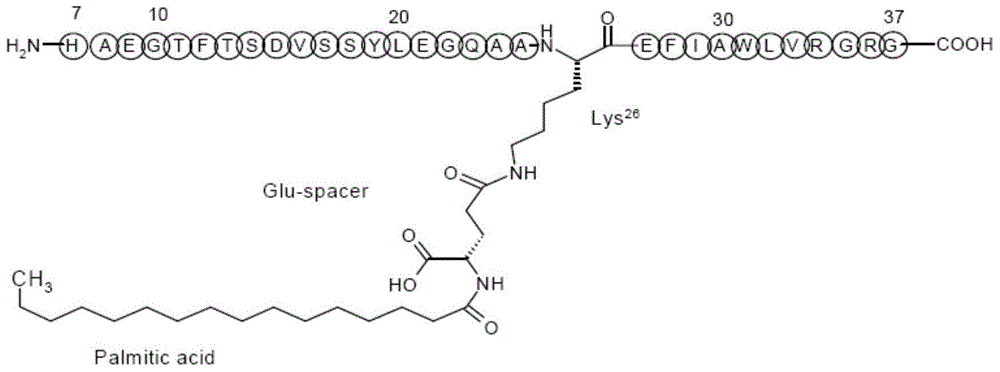
The invention discloses a polypeptide drug preparation with a mixture of citric acid and carbonate as a buffer salt system. The preparation comprises a GLP-1 analog, a buffer agent, an isotonic adjusting agent or a stabilizer and a preservative. The polypeptide drug preparation has the advantages that the pH (potential of hydrogen) of the polypeptide drug preparation is stabilized at a high level with a little amount of buffer salt, meanwhile, the buffer salt can reduce aggregation, degradation, oxidation and sedimentation of the GLP-1 analog, and the stability of the drug preparation is guaranteed in the transportation and storage processes. The invention further discloses a method for preparing the polypeptide drug preparation.
Description
technical field [0001] The present invention belongs to the field of GLP-1 analog pharmaceutical preparations, in particular to GLP-1 analog pharmaceutical preparations prepared in a buffer salt system composed of citrate and carbonate, more specifically to liraglutide pharmaceutical preparations and its preparation method. Background technique [0002] Glucagon-like peptide-1 (GLP-1) is a glucose-dependent incretin hormone secreted by L cells of the terminal jejunum, ileum, and colon. GLP-1 has multiple physiological effects on the human body: first, GLP-1 can act on the β cells of the pancreas, induce insulin secretion in a glucose concentration-dependent manner, improve the sensitivity of β cells, and promote the synthesis of insulin; it has also been found by animal experiments GLP-1 can increase the amount of β cells; GLP-1 can also act on α cells and inhibit the secretion of glucagon. Therefore, GLP-1 can effectively reduce blood sugar without increasing the occurrenc...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K38/26A61K47/12A61K47/02A61P3/10A61P3/04A61P25/28A61P9/00
Inventor 李霞黄河青谭永利张明会
Owner QILU PHARMA
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com