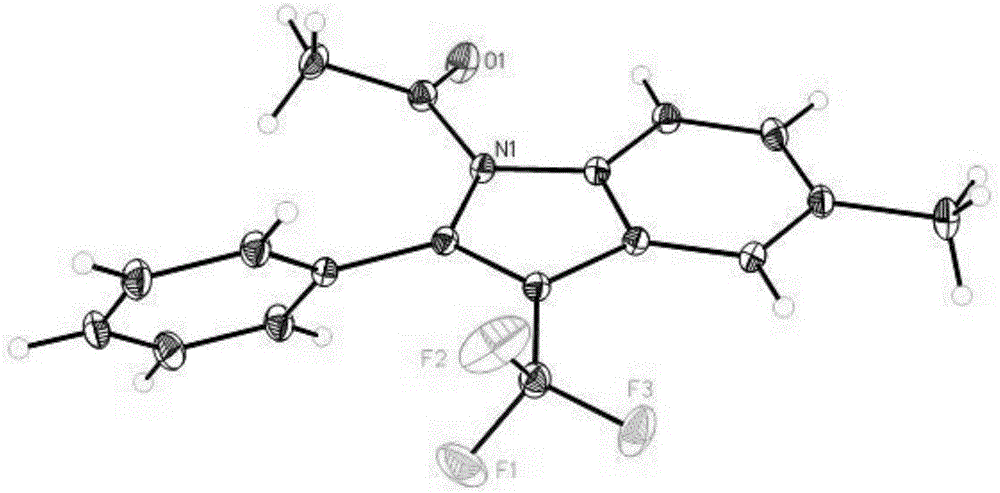
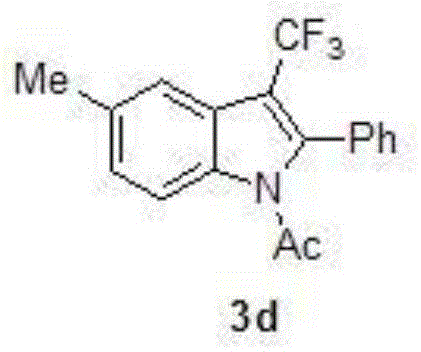
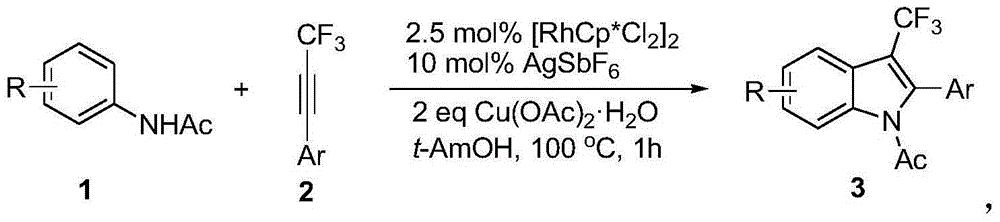Preparation method for 3-substituted trifluoromethyl indole
A technology of trifluoromethyl and trifluoromethylbenzene, applied in the field of preparation of 3-position trifluoromethyl-substituted indole, which can solve the problem of low reaction regioselectivity, limited practical use value, difficulty in obtaining o-iodoaniline, etc. problem, to achieve good application prospects, excellent chemical selectivity, and excellent reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021]
[0022] In the reaction test tube, add acetanilide successively, catalyst dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer (2.5mol%), oxidant copper acetate (2.0equiv), additive hexafluoroantimonic acid Silver (10mol%AgSbF 6 ) and solvent tert-amyl alcohol (2 mL), and finally trifluoromethylphenylacetylene (2.0 equiv) was added, and the reaction tube was sealed with a rubber stopper. Put the test tube oil bath in a 100°C oil bath with stirring and heating for about 1 hour. During the reaction, TLC was used to detect that the reaction was complete. In the post-treatment, the solvent was spin-dried first, and the pure product N-acetyl-3-trifluoromethyl-2-phenyl-indole compound 3a was directly separated by silica gel column chromatography. Yield: 81%; yellow solid, melting point 73-75°C; 1 HNMR (400MHz, CDCl 3 ,25℃,TMS):δ8.26(1H,d,J=8.4Hz),7.67(1H,d,J=8.0Hz),7.42-7.32(5H,m),7.30-7.25(2H,m) ,1.78(3H,s); 13 CNMR (100MHz, CDCl 3 ,25℃,TMS):δ171.2,138.6(q,J ...
example 2
[0025]
[0026] N-acetyl-3-trifluoromethyl-2-phenyl-7-methyl-indole, yield: 50%; yellow solid, melting point 117-120°C; 1 HNMR (400MHz, CDCl 3 ,25℃,TMS):δ7.58(1H,d,J=7.6Hz),7.44-7.36(5H,m),7.19(1H,t,J=7.6Hz),7.10(1H,d,J=7.6Hz) 7.6Hz), 2.27(3H,s), 1.92(3H,s); 13 CNMR (100MHz, CDCl 3 ,25℃,TMS):δ173.2,138.0(q,J C-F =4.1Hz), 134.5, 130.2, 129.9, 128.6, 127.9, 125.6, 123.8 (q, J C-F =267.4Hz), 123.7, 123.6, 117.7, 117.6, 108.9 (q, J C-F =34.8Hz), 28.7, 20.9; 19 FNMR (376MHz, CDCl 3 ,25℃,TMS):δ-54.21; HRMS(ESI):m / z[M+Na] + calcdfor(C 18 h 14 f 3 NO) Na: 340.0920; found: 340.0929.
example 3
[0028]
[0029] N-acetyl-3-trifluoromethyl-2-phenyl-6-methyl-indole, yield: 62%; yellow solid, melting point 92-94°C; 1 HNMR (400MHz, CDCl 3 ,25℃,TMS):δ8.11(1H,s),7.55(1H,d,J=8.0Hz),7.41-7.35(5H,m),7.11(1H,d,J=8.0Hz),2.42 (3H,s),1.78(3H,s); 13 CNMR (100MHz, CDCl 3 ,25℃,TMS):δ171.4,137.9(q,J C-F =4.1Hz), 136.4, 136.3, 131.2, 130.3, 129.9, 128.6, 125.9, 123.6 (q, J C-F =267.6Hz), 122.7, 119.2(d, J C-F =1.1Hz), 116.1, 111.7(q, J C-F =34.5Hz), 27.6, 21.9; 19 FNMR (376MHz, CDCl 3 ,25℃,TMS):δ-54.47; HRMS(ESI):m / z[M+Na] + calcdfor(C 18 h 14 f 3 NO) Na: 340.0920; found: 340.0926.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com