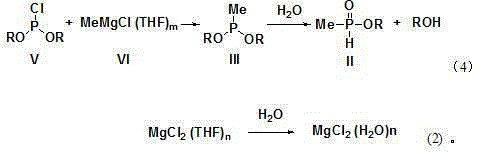
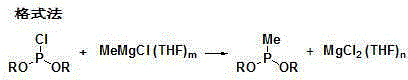Synthesis and purification method of methyl phosphinate compounds
A technology of methyl phosphonite and dialkyl methyl phosphonite, which is applied in the field of synthesis and purification of methyl phosphonite compounds, can solve problems such as unrealistic industrialization, and achieve the reduction of methyl phosphonite The formation, excellent reaction yield and product quality, the effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of embodiment 1 diethyl methylphosphonite
[0062] Under the protection of nitrogen, add 15.6g (0.1mol) of diethyl phosphonite chloride and 30mL tetrahydrofuran into a four-necked flask, start stirring and cool down to -10°C, add dropwise a tetrahydrofuran solution of methylmagnesium chloride (20%, 41.1 g, 0.11mol), keep the temperature at -10~-5°C, control the gas phase, diethyl phosphonite chloride ≤0.5%.
[0063] Control the temperature at -20~-15°C, add 40mL of 20% sodium chloride solution and 0.5g of hexadecyltributylphosphorus chloride to the above reaction solution, solids are precipitated, filter, and distill the filtrate under reduced pressure to collect 60~ 70°C / 50mmHg fraction, 10.15g of diethyl methylphosphonite was obtained, content (GC) 95%, 1 HNMR (500Hz, CDCl 3 ) δ 1.37(3H,t, J =7.0Hz), 1.55(3H, d, J =15.0Hz),4.15(2H, J =7.0, J =63.7Hz),7.23(1H,d, J =539Hz).
Embodiment 2
[0064] The synthesis of embodiment 2 ethyl methylphosphonite
[0065] Under the protection of nitrogen, add 15.6g (0.1mol) of diethyl phosphonite chloride and 30mL tetrahydrofuran into a four-necked flask, start stirring and cool down to -10°C, add dropwise a tetrahydrofuran solution of methylmagnesium chloride (22%, 37.4g , 0.11mol), keep the temperature at -10~-5°C, control the gas phase, diethyl phosphonite chloride ≤0.5%.
[0066] Control the temperature at -5~0°C, add 50mL of 15% sodium chloride solution and 0.3g of cetyltributylammonium chloride to the above reaction solution, solid precipitates, filter, distill the filtrate under reduced pressure, collect 55~60 °C / 90mmHg fraction, 8.8 g of ethyl methylphosphite was obtained, with a content (GC) of 96%.
Embodiment 3
[0067] The synthesis of embodiment 3 dibutyl methylphosphonite
[0068] Under the protection of nitrogen, add 21.2g (0.1mol) of dibutyl phosphonite chloride and 40mL tetrahydrofuran into a four-necked flask, start stirring and cool down to -15°C, add dropwise a tetrahydrofuran solution of methylmagnesium chloride (21%, 39.1 g, 0.11mol), keep the temperature at -10~-5°C, control the gas phase, dibutyl chloride phosphonite ≤0.5%.
[0069] Control the temperature at -15~-20°C, add 50mL of 25% magnesium chloride solution and 0.2g of hexadecyltributylammonium bromide to the above reaction solution, solid precipitates, filter, distill the filtrate under reduced pressure, collect at 70~80°C / 50mmHg fraction to obtain 12.75g of dibutyl methylphosphonite, content (GC) 96%, 1 HNMR (500Hz, CDCl 3 ) δ 0.95(3H,t, J =7.5Hz), 1.38(2H,m), 1.55(3H,d, J =15.5Hz), 1.70(2H,m), 4.05(2H, J =7.0, J =63.5Hz),7.22(1H,d, J =538Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com