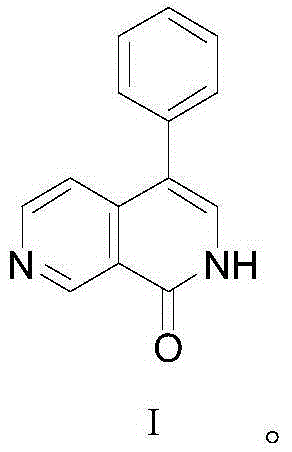
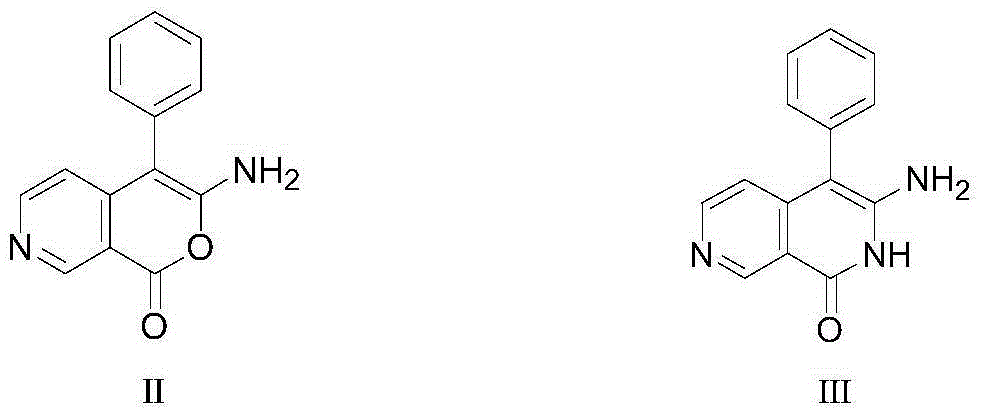Preparation method for 4-phenyl-2,7-naphthyridine-1(2H)-ketone
A technology of naphthyridine and phenyl, which is applied in the field of drug synthesis, can solve the problems of many route steps, low total yield, and limited output, and achieve the effects of low cost, low requirements for reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
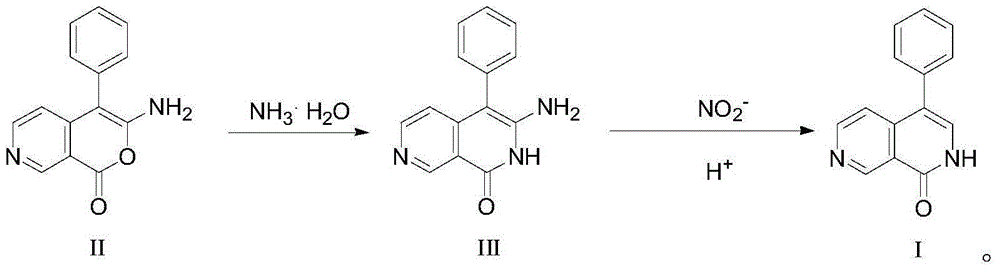
[0025] In a 100mL three-necked flask, add 2.8g (12mmol) of the raw material formula II compound 3-amino-4-phenyl-1H-pyran[3,4-c]pyridin-1-one, and then add 30mL of N,N-Dimethylformamide and 60mL concentrated ammonia water (equivalent to 70mmol of ammonia), then reacted at room temperature for 3h, then heated to 90°C for reflux reaction for 6h, after the reaction was completed, then use a rotary evaporator When steaming to a small amount of liquid, add 10mL of water and filter under reduced pressure to obtain 2.5g of yellow solid intermediate compound 3-amino-4-benzene-2,7-naphthyridin-1(2H)-one, the theoretical yield is 2.8g, the final yield mass yield is 89.3%.
[0026] Then take 0.8g (3.4mmol) of the intermediate product formula III compound 3-amino-4-benzene-2,7-naphthyridin-1(2H)-one obtained above and put it into another 100mL three-necked flask, and then add 20mL n-butanol solvent, 30mL of glacial acetic acid and 20mL of water, and then lower the temperature until the t...
Embodiment 2
[0038] In a 100mL three-necked flask, add 2.8g (12mmol) of the raw material formula II compound 3-amino-4-phenyl-1H-pyran[3,4-c]pyridin-1-one, and then add 40mL of THF solvent and 64mL concentrated ammonia water (equivalent to adding 75mmol of ammonia), then reacted at 30°C for 2.5h, then heated to 80°C for reflux reaction for 8h, after the reaction, steamed to an When there is a small amount of liquid, add 10mL of water and filter under reduced pressure to obtain 2.6g of yellow solid intermediate compound 3-amino-4-benzene-2,7-naphthyridin-1(2H)-one, the theoretical yield is 2.8g, The final yield mass yield is 92.9%.
[0039] Then take 0.8g (3.4mmol) of the intermediate product formula III compound 3-amino-4-benzene-2,7-naphthyridin-1(2H)-one obtained above and put it into another 100mL three-necked flask, and then add 30mL ethanol solvent, 50mL of hydrochloric acid aqueous solution with a mass concentration of 30%, and then lower the temperature until the temperature drops ...
Embodiment 3
[0042] In a 100mL three-necked flask, add 2.8g (12mmol) of the raw material formula II compound 3-amino-4-phenyl-1H-pyran[3,4-c]pyridin-1-one, and then add 40mL of Dioxane solvent and concentrated ammonia water, the addition of concentrated ammonia water is to make the amount of ammonia in concentrated ammonia water reach 24mmol, which is equivalent to making the compound of formula Ⅱ: the molar ratio of ammonia in concentrated ammonia water is 1:2, and then at 0 ℃ Under the conditions, the reaction was carried out for 3.5 hours, and then heated to 100°C for reflux reaction for 6 hours. After the reaction, evaporated with a rotary evaporator until there was a small amount of liquid, added 10mL of water and filtered under reduced pressure to obtain 2.55g of yellow solid intermediate product formula Compound III, 3-amino-4-benzene-2,7-naphthyridin-1(2H)-one, has a theoretical yield of 2.8 g and a final mass yield of 91.1%.
[0043] Then take 0.8g (3.4mmol) of the intermediate pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com