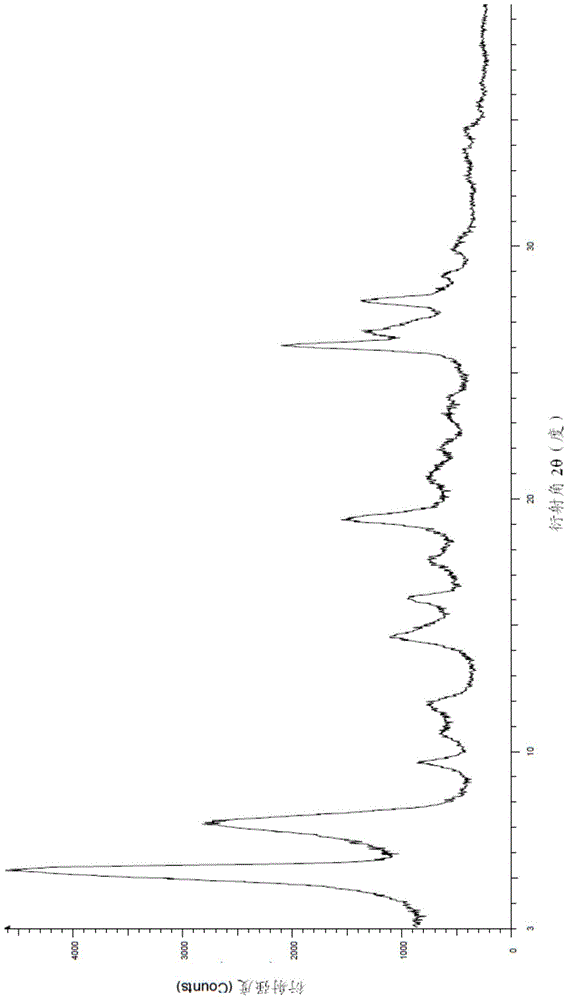
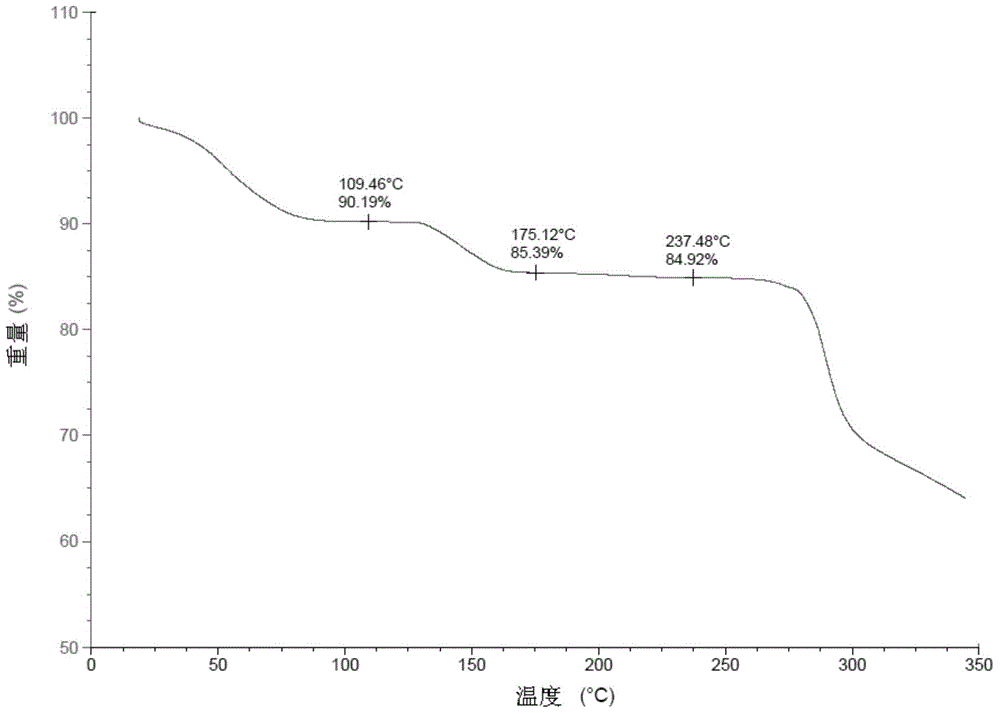
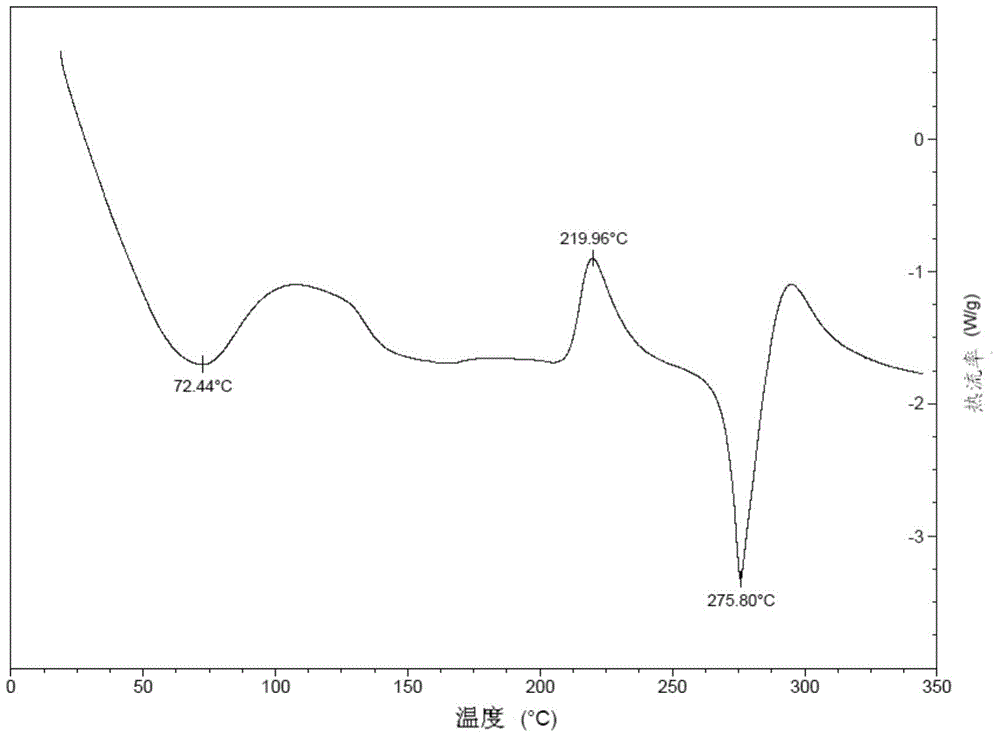
Preparation method of Regadenoson of crystal form B
A technology of regadesone and crystal form, which is applied in the field of preparation of regadesone crystal form B, can solve the problem of low purity of crystal form B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention discloses a preparation method of Ruijia Desson crystal form B, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all deemed to be included in the present invention. The method and application of the present invention have been described through the preferred embodiments. It is obvious that relevant personnel can modify or appropriately change and combine the methods and applications described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
[0036] The abbreviations used in the specification and claims and the specific meanings in English are as follows:
[0037]
[0038]
[0039] The raw materials or reagents used in the preparation...
Embodiment 1
[0046] Example 1 Preparation of Rega Desson Form B
[0047] Dissolve 40 mg of Regadesone in 2 mL of DMSO to obtain a 20 mg / mL solution. Filtered with a 0.45μm filter membrane, the liquid is injected into the 2mL quantitative loop of the reversed-phase chromatography system through the injection needle. The reversed-phase chromatographic conditions are: the column is C18, 250×4.6mm; the ultraviolet wavelength is 254nm; the mobile phase, phase B It is acetonitrile, phase A is the water phase, and the flow rate is 1 mL / min; the gradient elution method is adopted, and the specific gradient elution procedure is:
[0048] Time
B phase
0-5min
5%
5-10min
5%-10%
10-20min
15%
20-40min
15%-50%
40-45min
50%-90%
[0049] Separate according to the above established procedure, collect the main peak sample with a retention time of 15 minutes, and obtain a mixture of acetonitrile and water. 10 mL of dichloromethane was added to the obtained mixed solution, and the organic phase was...
Embodiment 2
[0058] Example 2 Preparation of Rega Desson Form B
[0059] Dissolve 10 mg of Regadesone in 1 mL of DMF to obtain a 27 mg / mL solution. Filtered with a 0.45μm filter membrane, the liquid is injected into the 2mL quantitative loop of the reversed-phase chromatography system through the injection needle. The reversed-phase chromatographic conditions are: the column is C18, 250×4.6mm; the ultraviolet wavelength is 254nm; the mobile phase, phase B It is acetonitrile, phase A is the water phase, and the flow rate is 1 mL / min; the gradient elution method is adopted, and the specific gradient elution procedure is:
[0060] Time
[0061] Separate according to the above established procedure, collect the main peak sample with a retention time of 15 minutes, and obtain a mixture of acetonitrile and water. 50 mL of ethyl acetate was added to the obtained mixed solution, and the organic phase was separated and concentrated under reduced pressure at 30°C. 1 mL of trifluoroethanol was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com