Purification method of clevidipine butyrate
A kind of technology of clevidipine butyrate and purification method, applied in the field of hypertension pharmaceutical, clevidipine butyrate purification, can solve the problem of reducing the product yield, deepening the color of the product, and the purification method does not mention the solution of genotoxic impurities, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
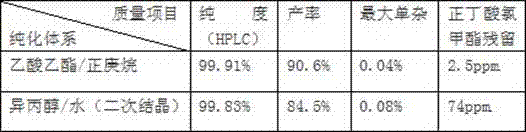
[0019] A purification method of clevidipine butyrate, adding 2.0L ethyl acetate to 0.500kg crude clevidipine butyrate, heating to reflux to dissolve, adding 3.0L of n-heptane in batches, heating to reflux to dissolve, system procedure Cool down, lower the temperature to 60°C and keep stirring for 0.5h, lower to 50°C and keep stirring for 0.5h, at this time the system starts to become turbid, naturally cool down to 40°C and keep stirring for 1h, then lower the temperature to 20°C and stir for 4h, filter, and filter the cake with acetic acid Wash with ethyl ester / n-heptane (2V / 3V) and dry to obtain the white product clevidipine butyrate 0.453kg with a yield of 90.6%, a purity (HPLC) of 99.91%, and a maximum of 0.04% chloromethyl butyrate Residual (GC) 2.5ppm.
[0020] The purification system of the embodiment of the present invention 1 and the effect comparison table of the purification system of the prior art:
[0021]
Embodiment 2
[0023] A purification method of clevidipine butyrate, adding 1.5L ethyl acetate to 0.500kg of crude clevidipine butyrate, heating to reflux to dissolve, adding 1.5L of n-heptane in batches, heating to reflux to dissolve, system procedure Cool down, lower the temperature to 60°C and keep stirring for 0.5h, lower to 50°C and keep stirring for 0.5h, at this time the system starts to become turbid, naturally cool down to 40°C and keep stirring for 1h, then lower the temperature to 20°C and stir for 4h, filter, and filter the cake with acetic acid Wash with ethyl ester / n-heptane (1V / 2V) and dry to obtain 0.442kg of white product clevidipine butyrate, with a yield of 88.4%, a purity (HPLC) of 99.85%, and a maximum of 0.05% mono-chloromethyl butyrate Residual (GC) 4ppm.
Embodiment 3
[0025] A purification method of clevidipine butyrate, adding 2.5L ethyl acetate to 0.500kg crude product of clevidipine butyrate, heating to reflux to dissolve, adding 5.0L of n-heptane in batches, heating to reflux to dissolve, system procedure Cool down, lower the temperature to 60°C and keep stirring for 0.5h, lower to 50°C and keep stirring for 0.5h, at this time the system starts to become turbid, naturally cool down to 40°C and keep stirring for 1h, then lower the temperature to 20°C and stir for 4h, filter, and filter the cake with acetic acid Wash with ethyl ester / n-heptane (1V / 2V) and dry to obtain 0.426kg of white product, clevidipine butyrate, with a yield of 85.2%, a purity (HPLC) of 99.90%, and a maximum of 0.04% chloromethyl butyrate. Residual (GC) 2ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com