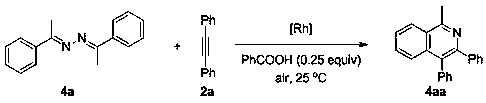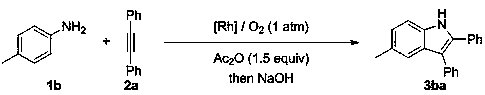Method for efficiently synthesizing indole and isoquinoline derivatives
A technology for isoquinoline and indole, which is applied in the field of efficient synthesis of indole and isoquinoline derivatives, can solve the problems of high temperature, low atom economy, and restriction on expansion of reaction substrates, and achieves clean reaction process and reduced Synthesis cost, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of indole derivative 3aa
[0041] Its synthetic route is as follows:
[0042]
[0043] Aniline 1a (55.0μL, 0.6mmol), 1,2-toluene 2a (71.3mg, 0.4mmol), Cp*Rh(H 2 O) 3 (OTf) 2 (11.8mg, 5mol%), acetic anhydride (59.0μL, 0.6mmol), added to 2.0mL pivalyl alcohol, under oxygen (1atm), 40 o After 24 hours of reaction in C, the reaction was stopped. After adding NaOH (48mg, 1.2mmol) and methanol 2mL and stirring for one hour, the pure product 2,3-diphenylindole 3aa was obtained by column chromatography. The product was a white solid with a yield of 70%.
[0044] 1 3 δ7.13-7.17(m,1H),7.21-7.45(m,12H),7.67(d, J =7.68,1H),8.18(br,1H);
[0045] 13 CNMR (100MHz, CDCl 3 )δ110.9, 115.0, 119.7, 120.4, 122.7, 126.2, 127.7, 128.1, 128.5, 128.7, 128.7, 130.1, 132.7, 134.0, 135.0, 135.9;
[0046] HRMS(EI)calcd.forC 20 h 15 N[M]:269.1204,found:269.1205.
Embodiment 2
[0047] Embodiment 2, the preparation of indole derivative 3aa
[0048] Aniline 1a (55.0μL, 0.6mmol), 1,2-toluene 2a (71.3mg, 0.4mmol), Cp*Rh(H 2 O) 3 (OTf) 2 (11.8mg, 5mol%), acetic anhydride (59.0μL, 0.6mmol), added to 2.0mL tert-butanol, under oxygen (1atm), 40 o After 24 hours of reaction in C, the reaction was stopped. After adding NaOH (48mg, 1.2mmol) and methanol 2mL and stirring for one hour, the pure product 2,3-diphenylindole 3aa was obtained by column chromatography. The product was a white solid in 18% yield. Data characterization is the same as in Example 1.
Embodiment 3
[0049] Embodiment 3, the preparation of indole derivative 3aa
[0050] Aniline 1a (55.0μL, 0.6mmol), 1,2-toluene 2a (71.3mg, 0.4mmol), Cp*Rh(H 2 O) 3 (OTf) 2 (11.8mg, 5mol%), acetic anhydride (59.0μL, 0.6mmol), added to 2.0mL acetone, under oxygen (1atm), 40 o After 24 hours of reaction in C, the reaction was stopped. After adding NaOH (48mg, 1.2mmol) and methanol 2mL and stirring for one hour, the pure product 2,3-diphenylindole 3aa was obtained by column chromatography. The product was a white solid with a yield of 49%. Data characterization is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com