Preparing method for 1-(N-alkylphenothizainyl)-1-methyl(H)-2-substituted Schiff base
A technology of alkylphenothiazine and methyl, which is applied in the field of preparation of 1--1-methyl-2-substituted Schiff base, can solve the problems of low yield, large amount of solvent used, long reaction time, etc. To achieve the effects of shortened reaction time, convenience and easy operation, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Add 0.005mol 3-acetyl-10-methylphenothiazine, 0.006mol thiosemicarbazide and 0.006mol p-toluenesulfonic acid to a dry mortar, and grind for 15min at room temperature. At this time, TLC monitoring shows that 3-acetyl The raw material point of -10-methylphenothiazine disappeared, indicating that the raw material was completely reacted, and then left to stand for 30 minutes to obtain a mixture; wherein the developer of TLC was a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0030] 2) After the mixture was washed with water and suction filtered, 1-(N-methylphenothiazinyl)-1-methyl-2-thiosemicarbazide Schiff base was obtained. m.p.: 162.6-163.7°C, yield 82%.
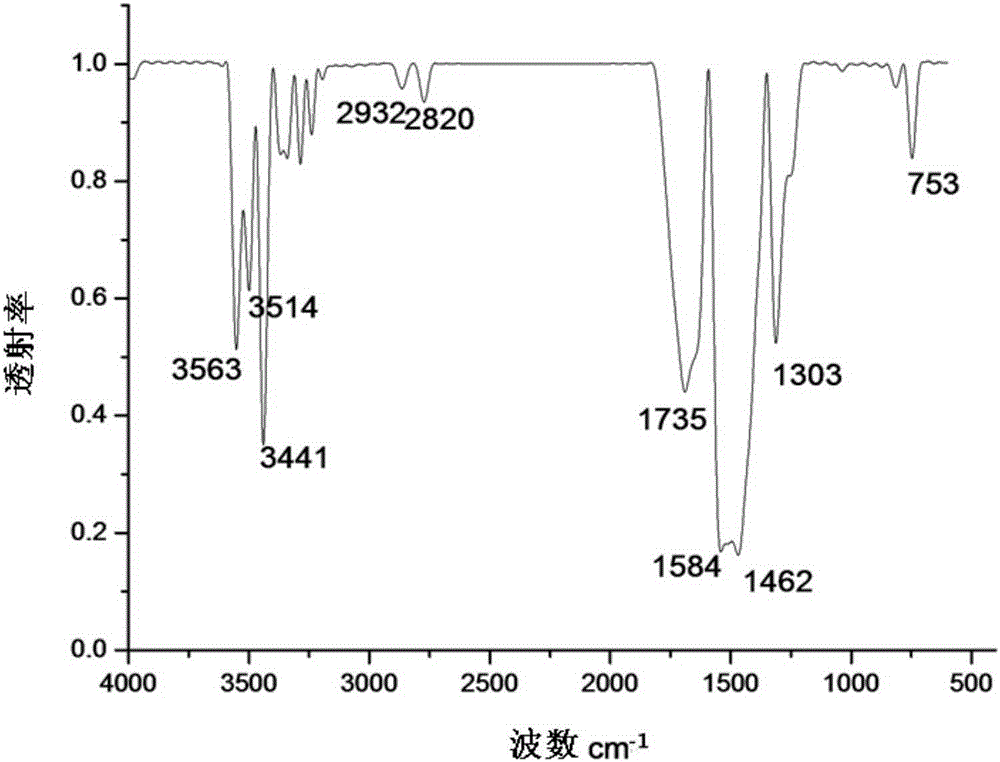
[0031] figure 1 For the infrared spectrum of the 1-(N-methylphenothiazinyl)-1-methyl-2-thiosemicarbazide Schiff base that embodiment 1 makes, by figure 1 Known: 3563cm -1 、3514cm -1 and 3441cm -1 is the N-H vibrational absorption peak, and at 2932cm -1 、1303cm -1 at -CH 3 T...
Embodiment 2
[0033] 1) Add 0.005mol 3-acetyl-10-ethylphenothiazine, 0.006mol semicarbazide hydrochloride and 0.006mol p-toluenesulfonic acid to a dry mortar, and grind for 16min at room temperature. At this time, TLC monitoring shows that 3-acetyl - The raw material point of 10-ethylphenothiazine disappeared, indicating that the raw material was completely reacted, and then left to stand for 30 minutes to obtain a mixture; wherein the developer of TLC was a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0034] 2) After the mixture was washed with water and suction filtered, 1-(N-ethylphenothiazinyl)-1-methyl-2-semicarbazide hydrochloride Schiff base was obtained. m.p.: 135.2-138.5°C, yield 86.5%.
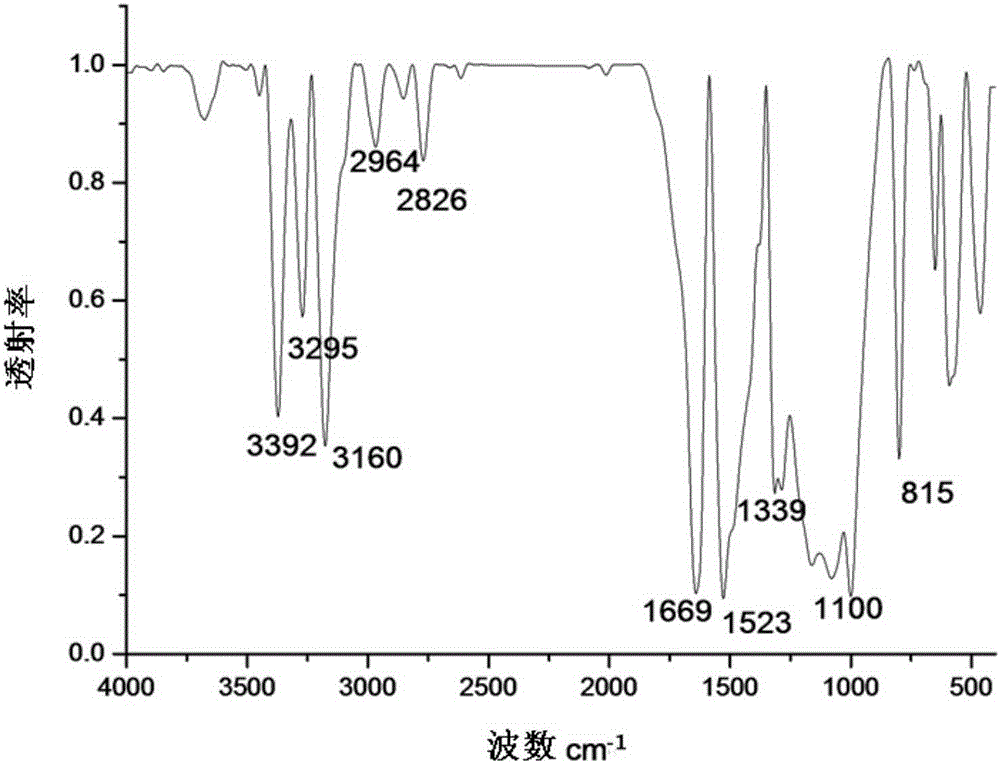
[0035] figure 2 For the infrared spectrum of the 1-(N-ethylphenothiazinyl)-1-methyl-2-hydrochloride semicarbazide Schiff base that embodiment 2 makes, by figure 2 Known: 3392cm -1 、3295cm -1 、3160cm -1 The vibrational absorption peak of N-H is at 2964cm -1...
Embodiment 3
[0037] 1) Add 0.005mol 3-formyl-10-methylphenothiazine, 0.006mol thiosemicarbazide and 0.006mol p-toluenesulfonic acid to a dry mortar, and grind for 15min at room temperature. At this time, TLC monitoring shows that 3-formyl The raw material point of -10-methylphenothiazine disappeared, indicating that the raw material was completely reacted, and then left to stand for 30 minutes to obtain a mixture; wherein the developer of TLC was a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0038] 2) After the mixture was washed with water and suction filtered, 1-(N-methylphenothiazinyl)-1-H-2-thiosemicarbazide Schiff base was obtained. m.p.: 174.2-176.5°C, yield 85.6%.
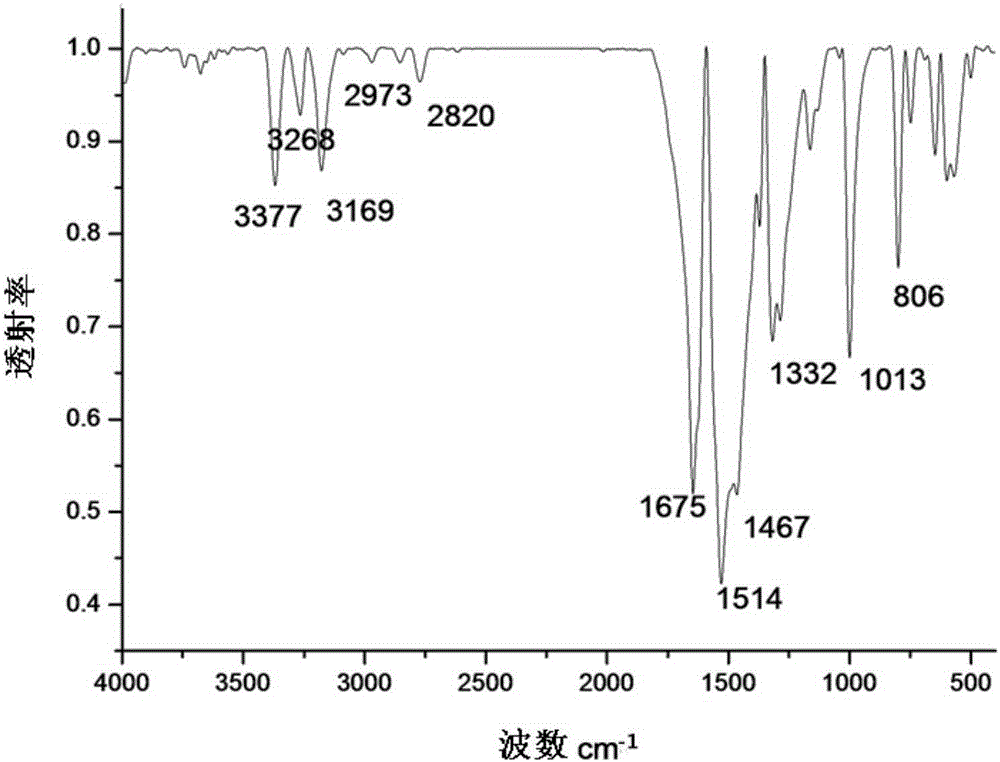
[0039] image 3 For the infrared spectrum of the 1-(N-methylphenothiazinyl)-1-H-2-thiosemicarbazide Schiff base that embodiment 3 makes, by image 3 Known: 3377cm -1 、3268cm -1 and 3169cm -1 The vibrational absorption peak of N-H is at 2973cm -1 、1332cm -1 at -CH 3 The absorpt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com