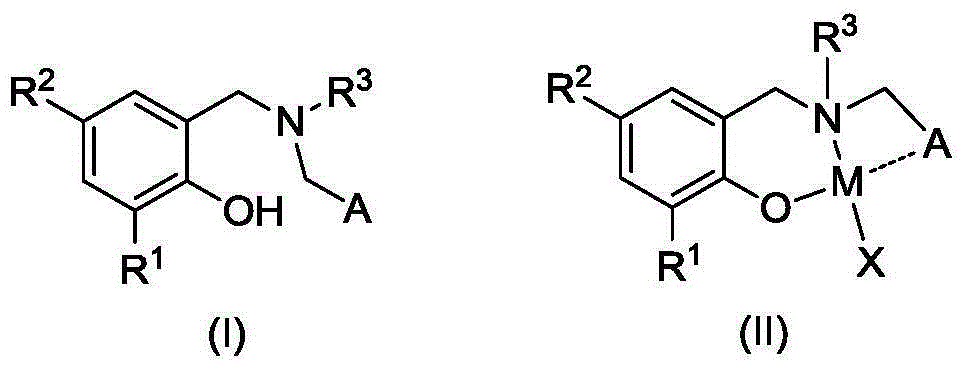
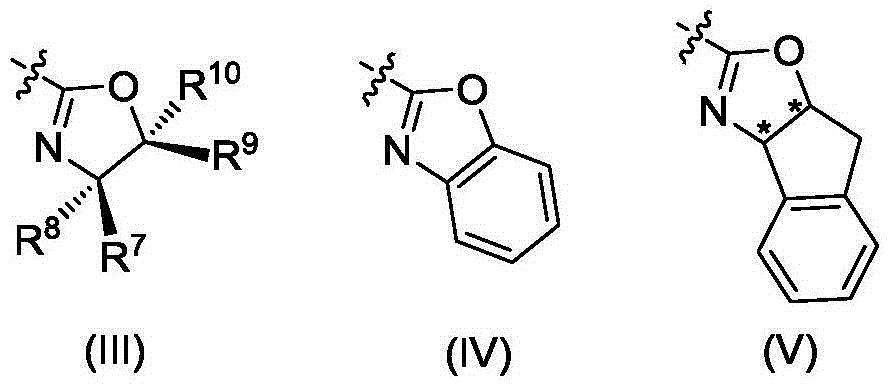
Oxazoline ring-containing amino tocopheroxyl zinc/magnesium complexes and preparation method and application thereof
A kind of aminophenoloxyzinc, oxazoline-containing technology, applied in magnesium organic compounds, zinc organic compounds, organic chemistry and other directions, can solve the problems of low selectivity, reduced catalytic activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
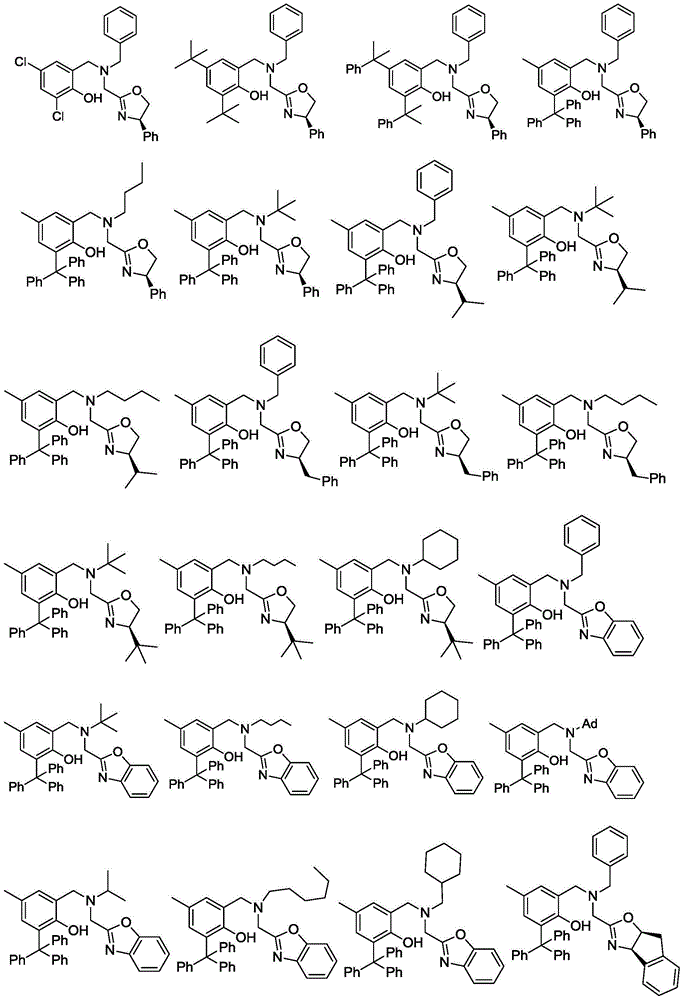
[0052] Synthesis of Ligand L1:
[0053] (1) Synthesis of N-[(4R)-2-methylene-4-phenyloxazoline]benzylamine
[0054]
[0055] Add benzylamine (8.21g, 76.6mmol) and potassium carbonate (1.17g, 8.47mmol) in the reaction flask, (4R)-2-chloromethyl-4-phenyl-2-oxazoline (1.50g, 7.66mmol ) and 25mLN,N-dimethylformamide, reacted for 12 hours. The solvent and unreacted benzylamine were distilled off under reduced pressure to obtain an orange-red oil. It is directly used in the next reaction, and the yield is calculated at 80%.
[0056] (2) Synthesis of Ligand L1
[0057]N-[(4R)-2-methylene-4-phenyloxazoline]benzylamine (2.23g, 6.70mmol), potassium carbonate (1.39g, 9.84mmol), 2-bromomethyl -4,6-dichlorophenol (2.55g, 9.96mmol) and 20mL N,N-dimethylformamide were reacted at room temperature for 2 hours. Add water to quench the reaction, extract with ethyl acetate, combine the organic phases, dry over anhydrous magnesium sulfate, filter, evaporate the solvent under reduced pressu...
Embodiment 2
[0061] Synthesis of Ligand L2
[0062] N-[(4R)-2-methylene-4-phenyloxazoline]benzylamine (1.50g, 4.51mmol), potassium carbonate (0.856g, 6.19mmol) and 2-bromomethyl- Except for 4,6-di-tert-butylphenol (1.11g, 3.71mmol), other operating steps were the same as in Example 1. Separation and purification by column chromatography gave light yellow foam L2 (1.01 g, 56%).
[0063]
[0064] 1 HNMR (CDCl 3 ,400MHz,298K):δ10.12(s,1H,OH),7.37–7.25(m,11H,ArH),6.91(d,1H, 4 J=2.4Hz, ArH), 5.23 (pesudot, 1H, 3 J=9.2Hz,CH 2 O),4.63(dd,1H, 3 J=8.4Hz, 2 J=10.4Hz,CH 2 O),4.12(t,1H, 3 J=8.4Hz, CHN), 4.00(s, 2H, ArCH 2 ),3.76(s,2H,PhCH 2 ),3.44(ddd,2H, 4 J=0.8Hz, 2 J=15.6Hz, 2 J=16.0Hz, NCH 2 C=N),1.46(s,9H,C(CH 3 ) 3 ),1.29(s,9H,C(CH 3 ) 3 ). 13 CNMR (CDCl 3 ,100MHz,298K):δ165.4,154.1,142.0,140.8,137.1,136.0,129.7,128.9,128.6,127.7,126.7,124.4,123.4,121.3,74.7,69.7,58.8,57.5,13.4,34.8,35. 29.8. Anal. Calcd. ForC 32 h 40 N 2 o 2 : C, 79.30; H, 8.32; N, 5.78. Found: C, 7...
Embodiment 3
[0066] Synthesis of Ligand L3
[0067] N-[(4R)-2-methylene-4-phenyloxazoline]benzylamine (4.95g, 14.9mmol), potassium carbonate (2.80g, 20.3mmol) and 2-bromomethyl- Except for 4,6-dicumylphenol (12.98g, calculated based on a yield of 50%, the actual raw material content is about 10.38g, 15.3mmol), the other operating steps are the same as in Example 1. Separation and purification by column chromatography gave light yellow foam L3 (2.12g, 23.4%).
[0068]
[0069] 1 HNMR (CDCl 3 ,400MHz,298K):δ9.79(s,1H,OH),7.34–7.11(m,19H,ArH),6.97-6.95(m,2H,ArH),6.79(d,1H, 4 J=2.0Hz, ArH), 5.15 (pesudot, 1H, 3 J=9.2Hz,CH 2 O),4.53(dd,1H, 3 J=8.4Hz, 2 J=10.4Hz,CH 2 O),4.02(t,1H, 3 J=8.4Hz, CHN), 3.84(s, 2H, ArCH 2 ),3.55(d,2H, 4 J=1.6Hz, PhCH 2 ),3.27(s,2H,NCH 2 C=N),1.69(s,12H,PhC(CH 3 ) 2 ). 13 CNMR (CDCl 3 ,100MHz,298K):δ165.0,153.6,151.6,151.5,142.0,140.2,136.8,135.5,130.0,128.8,128.5,128.0,127.8,127.6,127.5,126.9,126.6,126.5,125.5,125.8 121.5, 74.5, 69.5, 58.5, 56.5, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com