Matrix metalloproteinases and uses thereof
A matrix metal, protease technology, applied in the direction of peptide/protein components, enzymes, peptidases, etc., can solve problems such as instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
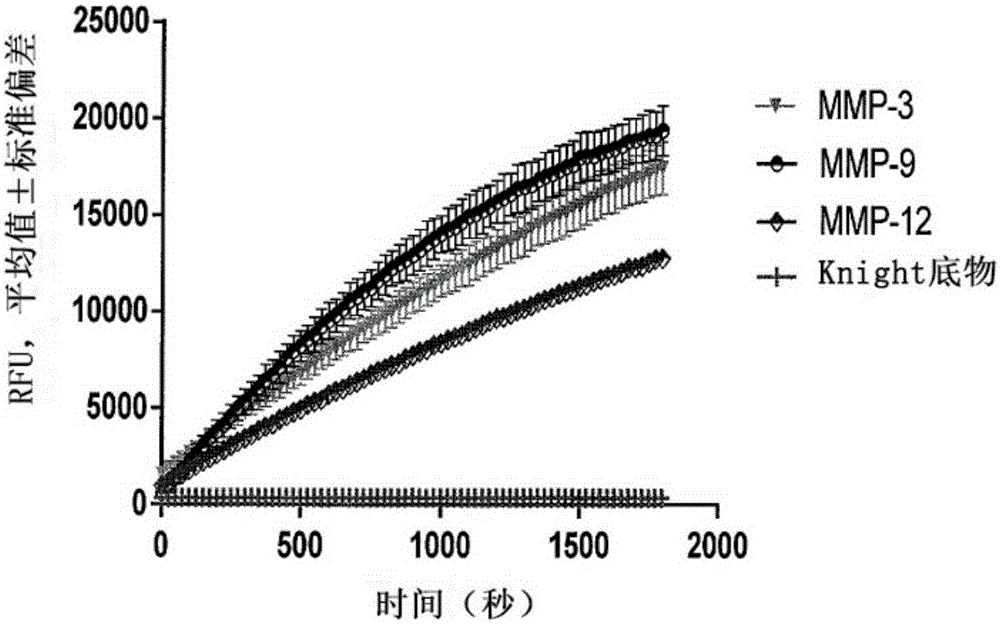
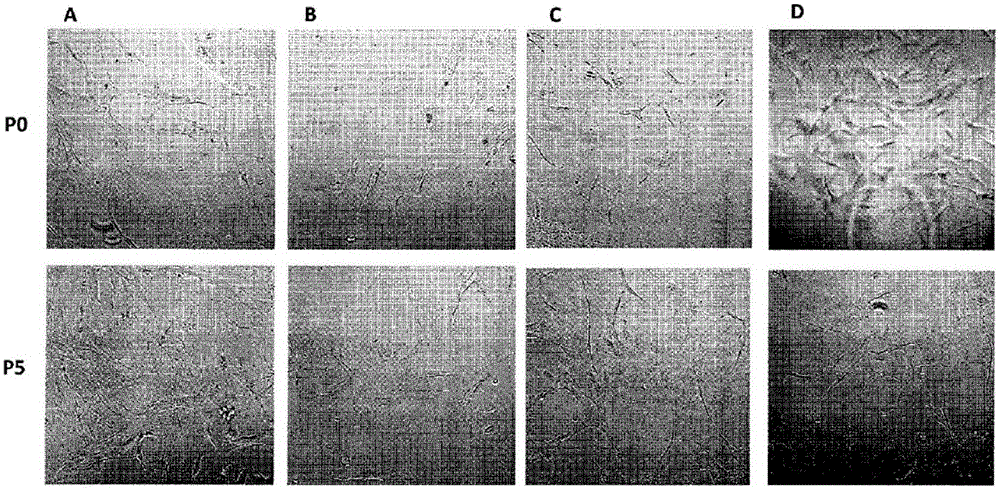
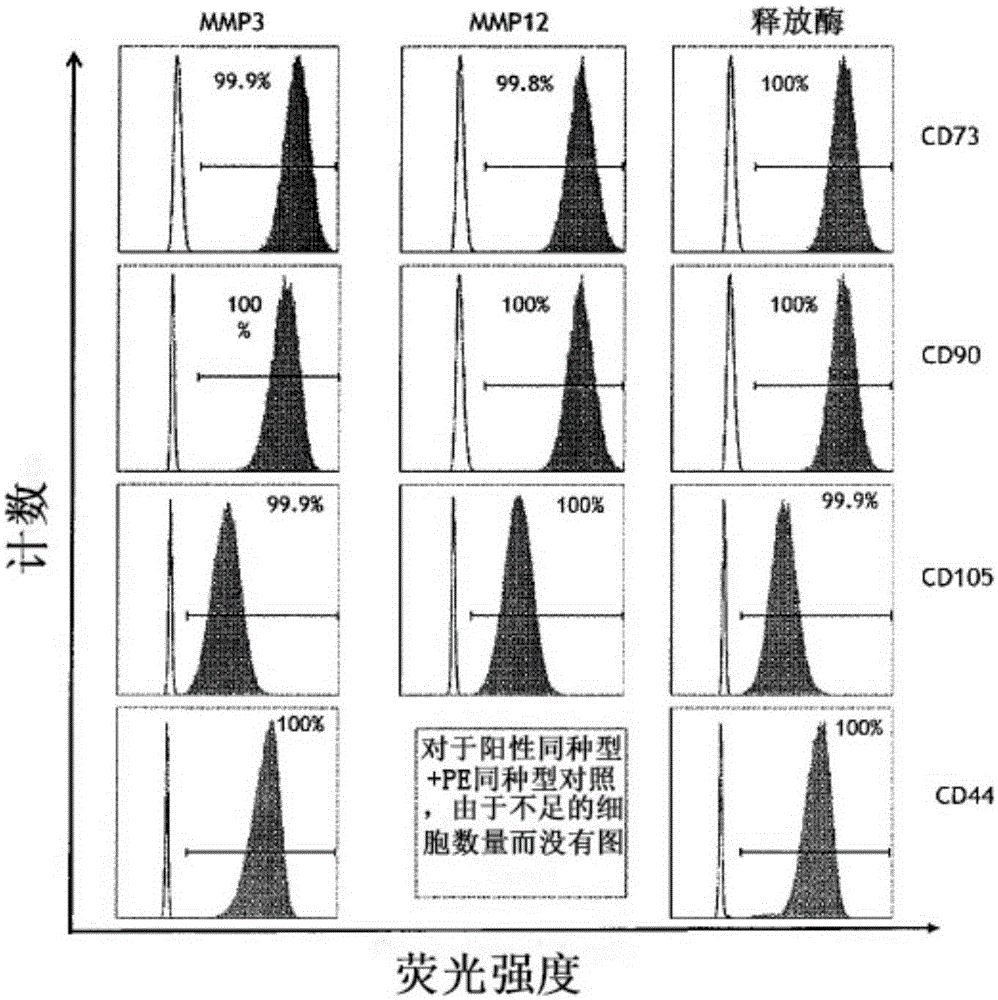
[0262] Example 1: MMP-induced isolation of stem cells from adipose tissue
[0263] Isolation of adipose-derived stromal stem cells (ADSCs) has mainly been achieved with collagenase I or liberase consisting of collagenases I and II and thermolysin (Priya, Sarcar et al., (2012) J. Tissue Eng. Regen . Med. Jul. 27:1-9). Collagenase types I and II were purified from the highly dangerous Clostridium spp., an agent of gas gangrene. Furthermore, crude preparations from Clostridium histolyticum contained not only some collagenases, but also thiol proteases, clostripain, trypsin-like enzymes and aminopeptidases. During the release of the enzyme production, the collagenase isoenzyme is purified by a process of removing the considerable amount of endotoxin present in the raw material. There was a wide range of endotoxin contamination of conventional collagenase preparations compared to endotoxin levels of releasing enzymes. However, regardless of the source, all purified collagenases ...
Embodiment 2
[0288] Example 2: Separation of various cells
[0289] Islet isolation from pancreas: There are three main steps: in situ perfusion of the pancreas with an MMP-mixture (eg two or more MMPs), digestion of the pancreas and purification of the islets.
[0290] Mice are first anesthetized with pentobarbital or the like and then moved into a hood. Each mouse was laid down, belly side up, and its skin was disinfected with 70% ethanol. An incision is made around the upper abdomen to expose the liver and intestines. Clamp the ampulla of the mouse against the duodenal wall with surgical forceps to block the path of bile to the duodenum. Prepare 3 ml of buffer by dissolving the MMP mixture in 5 ml of 1X HBSS and draw into a 5 ml syringe fitted with a 30G1 / 2-G needle. The needle is then inserted under a microscope into the common bile duct through the junction of the hepatic and cystic ducts and into the middle of the common bile duct. The solution is injected slowly to expand the panc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com