Chinese herbal medicine powder-decocting feed additive formula for medium-stage culture of prawns
A technology for feed additives and Chinese herbal medicines, which is applied to the field of Chinese herbal medicine boiled and scattered feed additive formulations in the mid-term shrimp culture, can solve the problems of high breeding risks, influence on shrimp growth, and shrimp incompatibility, so as to enhance the ability to resist stress and improve the ability to resist diseases. , the effect of improving the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The invention discloses a feed additive formula of Chinese herbal medicine boiled in the middle stage of prawn cultivation, which is composed of the following raw materials in parts by weight: 8 parts of Luo Han Guo, 12 parts of honeysuckle, 12 parts of Polygonum cuspidatum, 15 parts of Astragalus membranaceus, 15 parts of Fangfeng and 25 parts of tangerine peel.
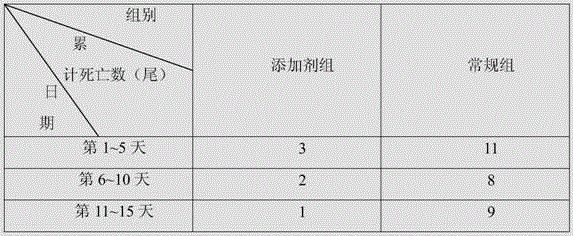
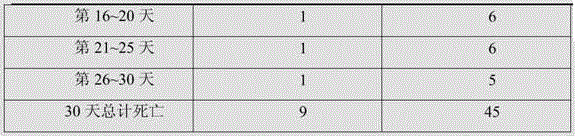
[0018] Adopt additive of the present invention to be used for the test of culture pond of Penaeus monodon, test result is shown in Table 1.
[0019] In this experiment, the additive group and the conventional group were set up. The culture ponds used in the additive group and the conventional group were adjacent to each other. The culture pond had a clear area of one square meter, a water depth of one meter, the same water quality, and the same external environment. The same batch of Penaeus monodon fed in the same shrimp pond for 30 days was used and divided into two breeding ponds, each with 150 tails. Th...
Embodiment 2
[0025] The invention discloses a feed additive formula of Chinese herbal medicine boiled in the mid-term culture of prawns, which consists of the following raw materials by weight: 12 parts of Luo Han Guo, 8 parts of honeysuckle, 8 parts of knotweed, 25 parts of astragalus, 25 parts of Fangfeng and 15 parts of tangerine peel.
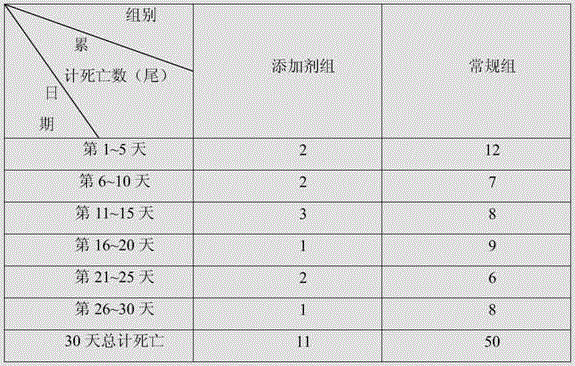
[0026] Adopt additive of the present invention to be used for the test of penaeus prawn culture pond, test result is shown in Table 2.
[0027] In this experiment, the additive group and the conventional group were set up. The culture ponds used in the additive group and the conventional group were adjacent to each other. The culture pond had a clear area of one square meter, a water depth of one meter, the same water quality, and the same external environment. The same batch of Penaeus prawns fed in the same pond for 30 days was used, and they were divided into two breeding ponds, each with 150 tails. The additive group was fed with the additive comp...
Embodiment 3
[0032] The invention discloses a feed additive formula of Chinese herbal medicine boiled in the mid-term culture of prawns, which consists of the following raw materials in parts by weight: 10 parts of Luo Han Guo, 10 parts of honeysuckle, 10 parts of Polygonum cuspidatum, 20 parts of Astragalus membranaceus, 20 parts of Fangfeng, and 20 parts of tangerine peel.
[0033] Adopt the additive of the present invention to be used for the penaeus vannamei breeding pond test, and the test results are shown in Table 3.
[0034] In this experiment, the additive group and the conventional group were set up. The culture ponds used in the additive group and the conventional group were adjacent to each other. The culture pond had a clear area of one square meter, a water depth of one meter, the same water quality, and the same external environment. The same batch of Penaeus vannamei fed in the same shrimp pond for 30 days was used and divided into two breeding ponds, each with 150 tails. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com