Injectable cartilage tissue protection and repair material containing xanthan gum
A cartilage tissue and repair material technology, applied in the field of medicine, can solve the problems of poor thermal stability of medical chitosan, high temperature resistance of injections, slow repair speed, etc., to achieve the benefits of sports penetration, good protection of cartilage tissue, and long-term action long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
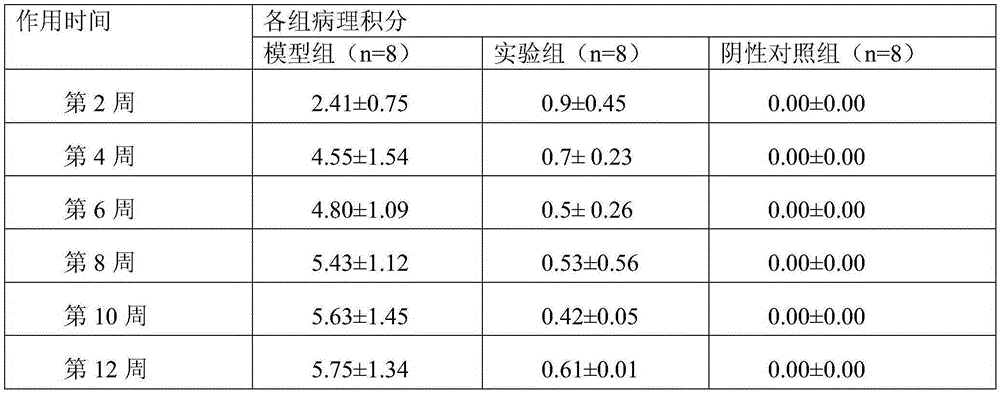
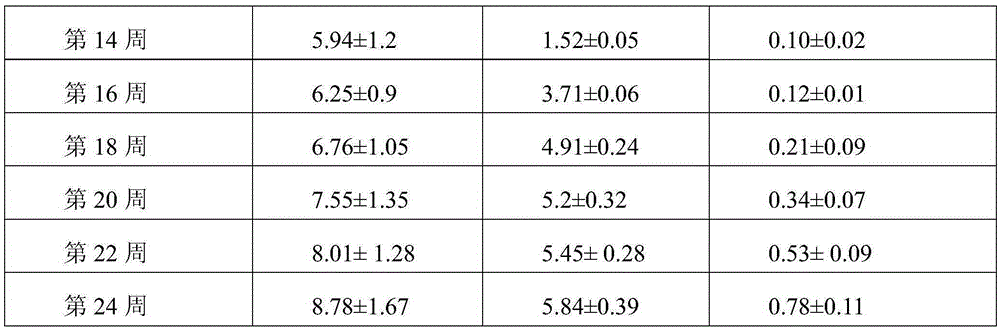
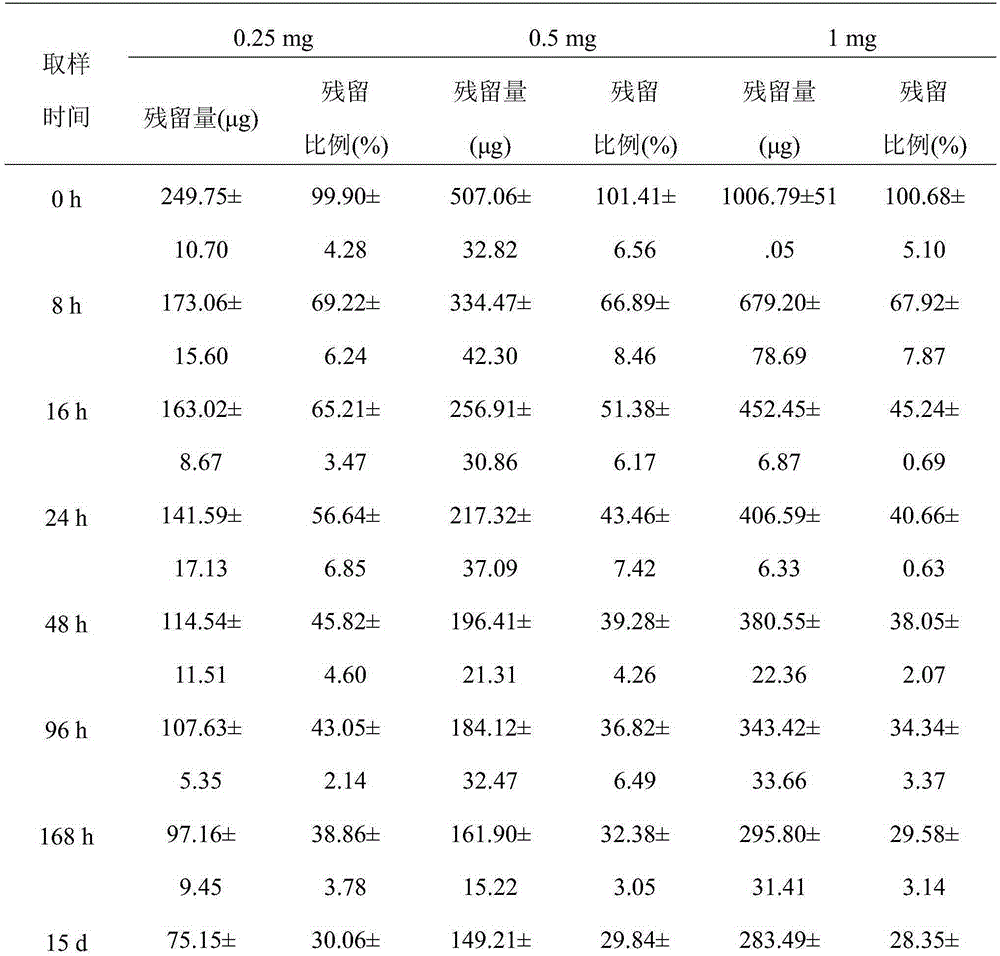
[0031] An injectable cartilage tissue protection and repair material containing xanthan gum, consisting of the following components: xanthan gum 10g, disodium hydrogen phosphate 1g, sodium dihydrogen phosphate 10g and water for injection 500g; the xanthan gum The average relative molecular mass is 2.1 million.
Embodiment 2
[0033] An injectable cartilage tissue protection and repair material containing xanthan gum, which is composed of the following components: 1 g of xanthan gum, 1 g of disodium hydrogen phosphate, 10 g of sodium dihydrogen phosphate and 500 g of water for injection; The average relative molecular mass is 2.5 million.
Embodiment 3
[0035] An injectable cartilage tissue protection and repair material containing xanthan gum, consisting of the following components: xanthan gum 10g, disodium hydrogen phosphate 1g, sodium dihydrogen phosphate 10g and water for injection 500g; the xanthan gum The average relative molecular mass is 3 million.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com