ACVR1-Fc fusion protein, and preparation method and application thereof
A technology of fusion protein and protein, applied in the field of biotechnology and medicine, can solve the problem of low long-term survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1. Construction of Fusion Protein Expression Plasmid
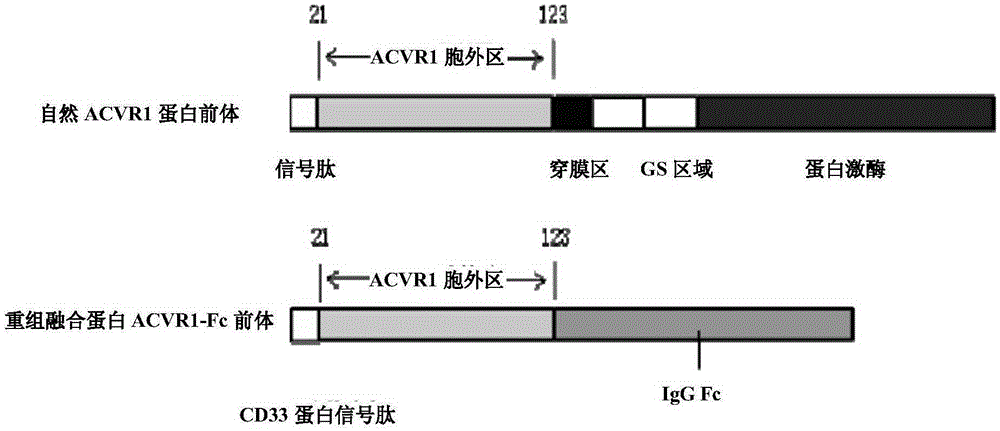
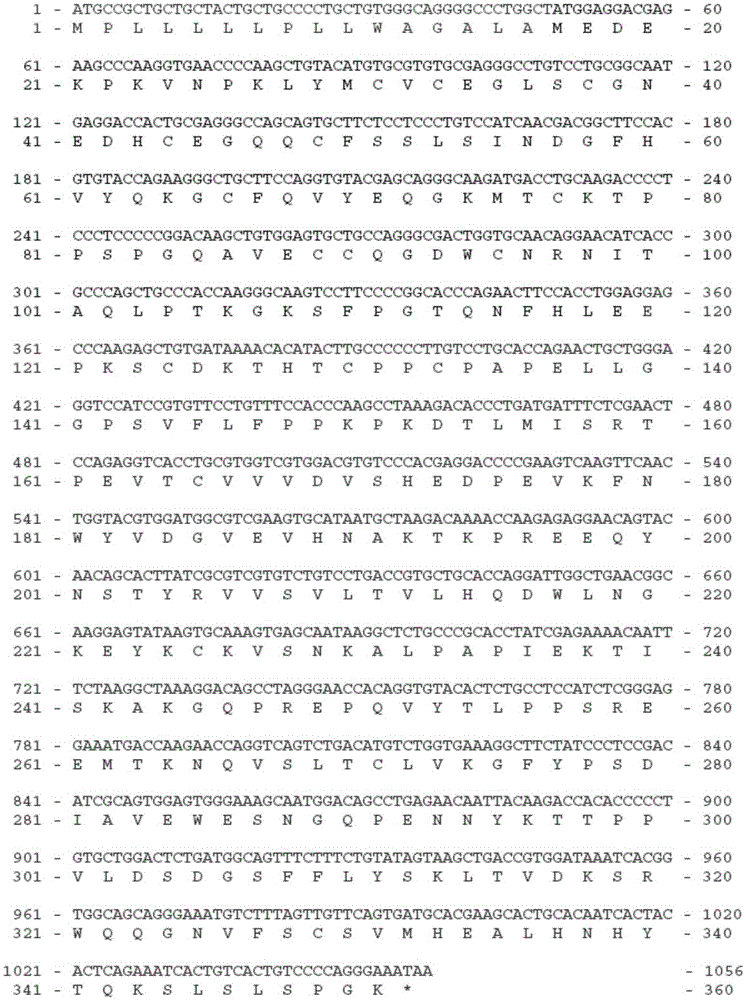
[0179] ACVR1-Fc expression gene consists of 3 fragments (such as image 3 shown), from the 5' end to the 3' end as follows:
[0180] Fragment 1: the signal peptide sequence of the protein CD33 located at the 5' end (its coding sequence is shown in SEQ ID NO: 1, and its amino acid sequence is shown in SEQ ID NO: 2);
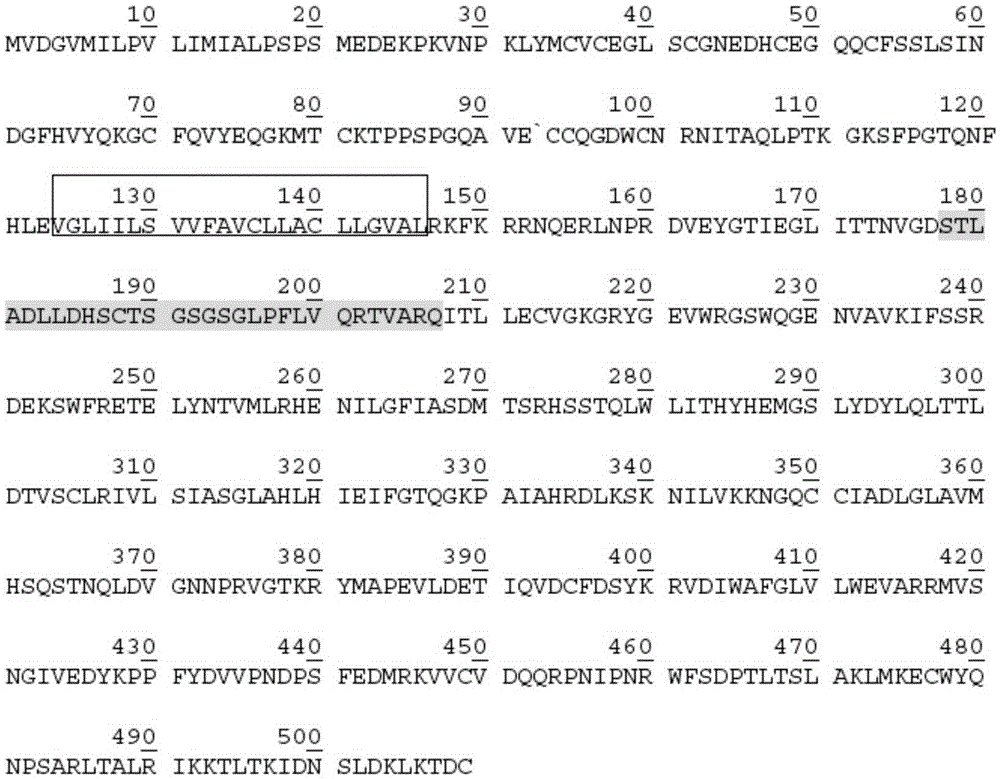
[0181] Fragment 2: the expression gene of amino acids 21-123 of the extracellular segment of ACVR1 located in the middle (its coding sequence is shown in SEQ ID NO: 3, and its amino acid sequence is shown in SEQ ID NO: 4);
[0182] Fragment 3: the coding sequence of the human IgGγ1 amino acid sequence at the 3' end (the coding sequence is shown in SEQ ID NO: 5, and the amino acid sequence is shown in SEQ ID NO: 6), which encodes amino acids from positions 216 to 447 of human IgGγ1 Residues, including the hinge region and the second and third CH regions (ie hinge region+CH2+CH3).
[0183] Th...
Embodiment 2
[0207] Example 2. Establishment of fusion protein expression cell lines
[0208] The host cell CHODG44 was derived from (purchased from Invitrogen Corporation, USA, Cat. No. 12609-012), and the method of cell culture and passage was referred to the CHODG44 manual of the company. Non-transfected cells were cultured in suspension in CDDG44 medium (Invitrogen) containing 8 mM L-glutamine and 5 μg / ml recombinant human insulin.
[0209] The CHODG44 cell line with stable and high-efficiency protein expression was established by stable transfection method. The cloned CHODG44 cells were cultured in suspension in serum-free, animal protein-free medium.
[0210] The method and steps of constructing a stable expression cell line of the fusion protein are briefly described as follows: use TianGen’s plasmid extraction kit to prepare the fusion protein expression vector plasmid, and digest 100 μg of the plasmid with the restriction endonuclease PuvI to make the plasmid linear. DG44 cell...
Embodiment 3
[0212] Example 3. Protein A affinity chromatography and HPLC-SEC analysis of fusion proteins
[0213] The ACVR1-Fc fusion protein was purified from the culture supernatant of stably expressing cells by protein A affinity chromatography. The purification method refers to the standard protein A (POROS, MabcaptureA) affinity chromatography method, and the purified protein is analyzed by reducing and non-reducing SDS-PAGE electrophoresis, and HPLC-SEC (high pressure liquid phase-molecular sieve) analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com