Method for preparing ultralight manganese dioxide aerogel
A manganese dioxide and aerogel technology, applied in manganese oxide/manganese hydroxide and other directions, can solve the problems of residual organic modified molecules, high porosity, high specific surface area, etc., and achieve the effect of simple equipment and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Preparation of manganese dioxide nanosheets:
[0028] Dissolve 3.20mmol sodium lauryl sulfate in 32mL deionized water; then add 3.2mL of aqueous hydrochloric acid solution with a concentration of 100mM, 3.2mL of aqueous potassium permanganate solution with a concentration of 50mM and 281.6mL of deionized water; after mixing evenly Heated at 95°C for 3 hours to obtain dark brown precipitates of manganese dioxide nanosheets with a mass of ~12.5 mg;
[0029] 2) Preparation of ultrathin manganese dioxide nanosheet colloidal solution:
[0030] The manganese dioxide nanosheet precipitate prepared in step 1) was repeatedly washed with ethanol and deionized water for 3 times for purification, then 12.5 mL of deionized water was added to the precipitate, and the mixed solution was ultrasonicated for 30 minutes to obtain a brown supernatant. Colloidal solution of thin manganese dioxide nanosheets, the concentration is ~1.0mg / mL;
[0031] 3) Preparation of ultra-light mangane...
Embodiment 2
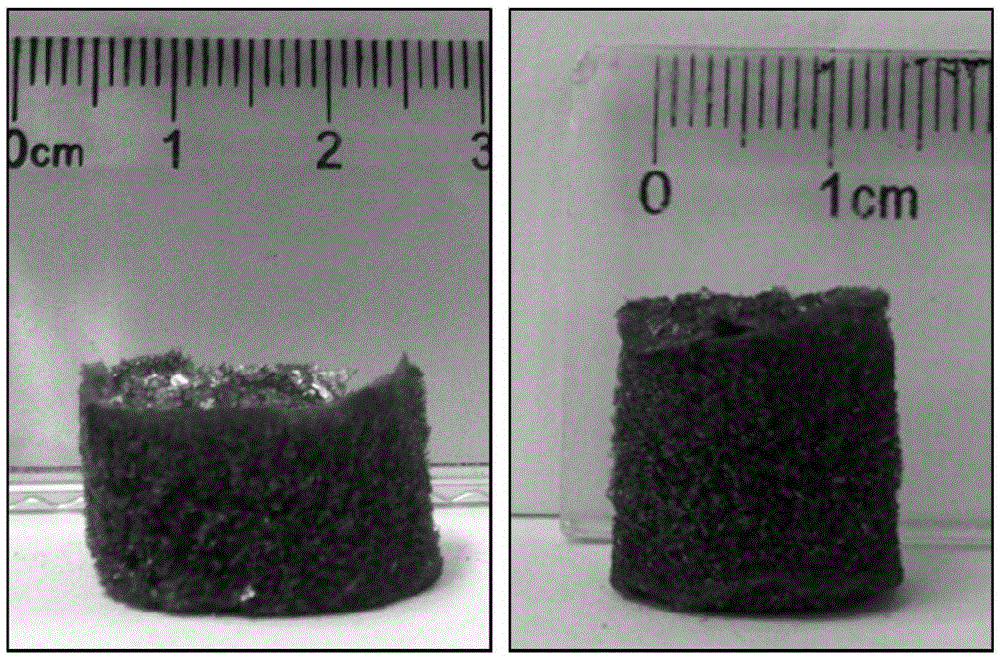
[0037] As in the steps of Example 1, the difference is that in step 2) of Example 1, the precipitated manganese dioxide nanosheets were rinsed repeatedly with ethanol and deionized water for 3 times, then 12.5 mL of deionized water was added, and the mixed solution was then ultrasonically In 30 minutes, a tan ultrathin manganese dioxide nanosheet colloidal solution was obtained, and the concentration was ~1mg / mL; while the volume of deionized water added in Example 2 was 25mL, and then the mixed solution was ultrasonicated for 30 minutes to obtain a tan ultrathin Colloidal solution of manganese dioxide nanosheets, the concentration is ~ 0.50mg / mL; further use the same method to prepare manganese dioxide airgel. The prepared manganese dioxide airgel is cylindrical with a volume of 3.3 cm 3 , with a mass of 1.7mg and a density of 0.51mg / cm 3 .
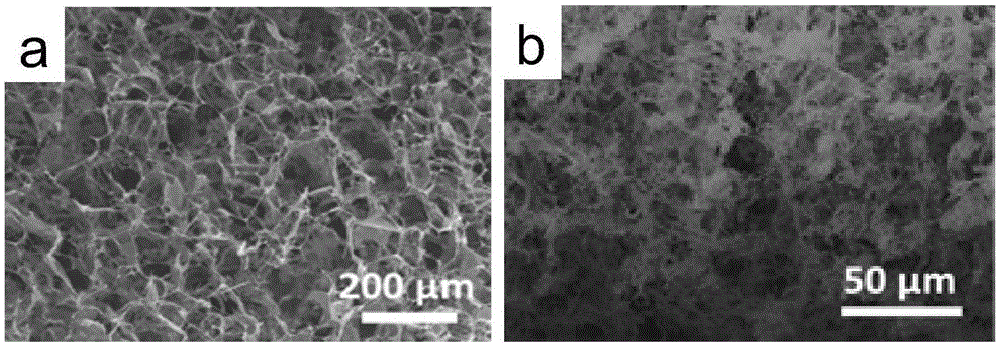
[0038] attached Figure 4 It is a scanning electron micrograph of the manganese dioxide airgel obtained in this example. As shown i...
Embodiment 3
[0040] As in the steps of Example 1, the difference is that in step 2) of Example 1, the precipitated manganese dioxide nanosheets were rinsed repeatedly with ethanol and deionized water for 3 times, then 12.5 mL of deionized water was added, and the mixed solution was then ultrasonically In 30 minutes, a tan ultrathin manganese dioxide nanosheet colloidal solution was obtained, and the concentration was ~1mg / mL; while the volume of deionized water added in Example 3 was 5mL, and then the mixed solution was ultrasonicated for 30 minutes to obtain a tan ultrathin Manganese dioxide nanosheet colloidal solution, the concentration is ~ 2.5mg / mL; further use the same method to prepare manganese dioxide airgel. The prepared manganese dioxide airgel is cylindrical with a volume of 3.3 cm 3 , with a mass of 8.3mg and a density of 2.5mg / cm 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
| Average length | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com