Method for preparing 2-dicyclohexylphosphine-2,4,6-di-iso-propylbiphenyl
A technology of triisopropylbiphenyl and dicyclohexylphosphine, applied in the field of preparing 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl, which can solve the problem of affecting product purity and quality, and scale-up production Inconvenient operation, troublesome post-processing and other problems, to achieve the effect of ensuring purity and quality, high yield, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
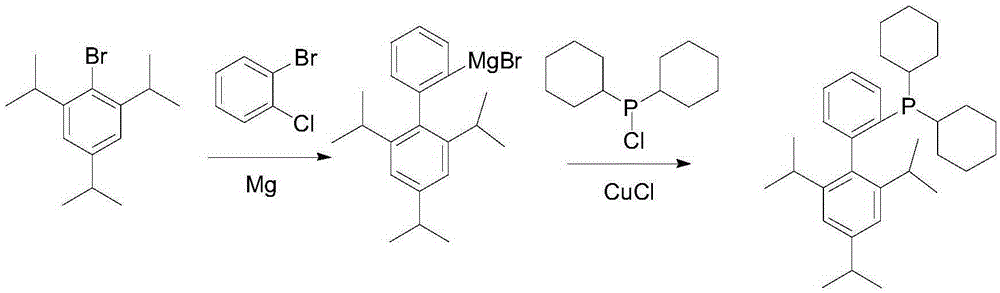
[0036] The invention provides a preparation method of 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl shown in formula (I), comprising:
[0037] Under the protection of an inert gas such as nitrogen, add butyllithium dropwise to 2-halo-2,4,6-triisopropylbiphenyl represented by formula (II) at -10-10°C, and react for 0.5-3 Hours, then drop dicyclohexylphosphine chloride at -10-10°C, naturally rise to room temperature and react for 1-5 hours, add saturated aqueous solution of weak acid and weak base salt to the reaction liquid in an ice-water bath to quench the reaction, and separate , The organic phase was precipitated, the solvent was removed, crystallized by adding alcohol, and filtered to obtain white 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl.
[0038] Among them, 2-halo-2,4,6-triisopropylbiphenyl is 2-chloro-2,4,6-triisopropylbiphenyl or 2-bromo-2,4,6-triisopropyl biphenyl. Butyllithium may be n-butyllithium, sec-butyllithium or tert-butyllithium, preferably n...
Embodiment 1
[0058] Under nitrogen protection, add 200mL of THF and 31g of 2,4,6-triisopropylbromobenzene into a 1L three-necked flask, drop to 0°C, add 46mL of 2.5M n-butyllithium dropwise, and react for 1 hour , 20g of o-chlorobromobenzene was added dropwise at 0°C, and the reaction was completed for 1 hour to generate 2-chloro-2,4,6-triisopropylbiphenyl;
[0059] Add 42mL of 2.5M n-butyllithium dropwise to the reaction solution at 0°C. After the dropwise reaction is completed for 1 hour, 26.7g of dicyclohexylphosphine chloride is added dropwise, and the reaction is naturally raised to room temperature for 2 hours. Add 200 mL of saturated ammonium chloride aqueous solution dropwise to the reaction liquid in an ice-water bath to quench, separate the liquid, desolventize the organic phase, add methanol to crystallize, and filter to obtain white 2-dicyclohexylphosphine-2,4,6-triiso Propyl biphenyl 45g, yield 90%.
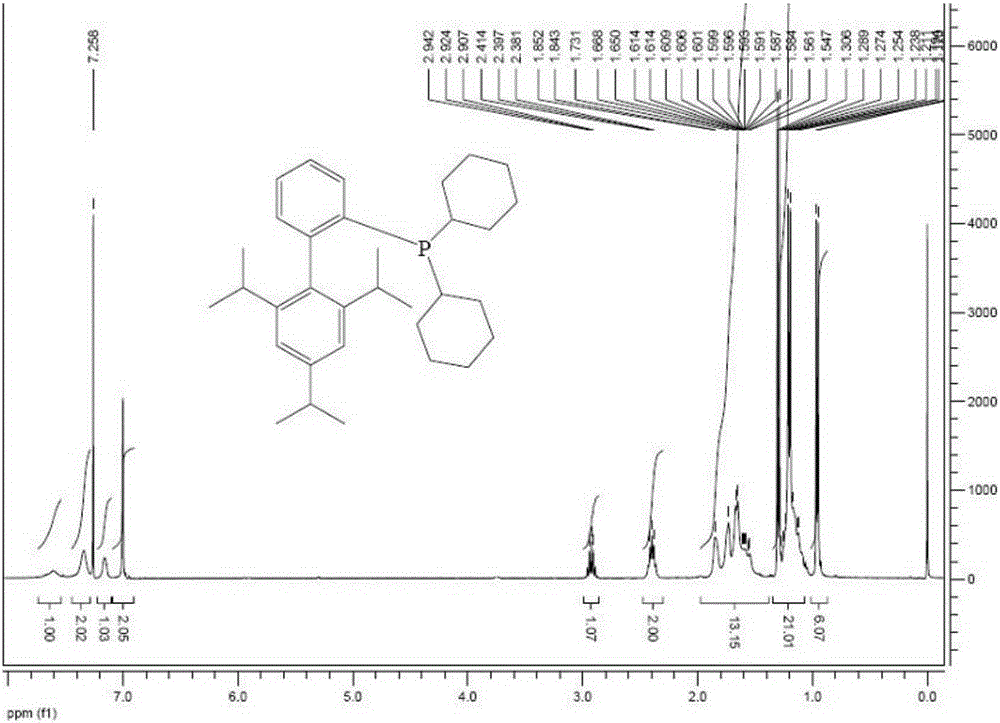
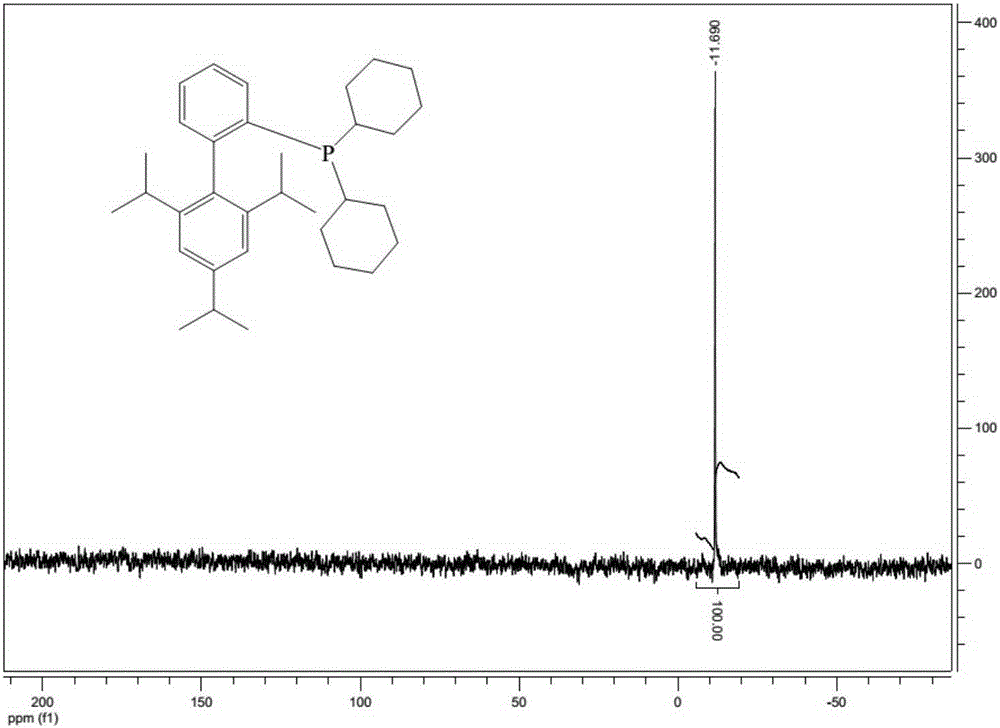
[0060] figure 1 and figure 2 are the prepared 2-dicyclohexylphosphine-2,...
Embodiment 2
[0065] Under nitrogen protection, add 200mL of anhydrous methyl tetrahydrofuran and 28g of 2,4,6-triisopropylbromobenzene to a 1L three-necked flask, drop to -10°C, add 1.0M sec-butyllithium 156mL dropwise, After the dropwise reaction for 30 minutes, 20 g of o-chlorobromobenzene was added dropwise at 10°C, and the reaction was completed for 20 minutes to generate 2-chloro-2,4,6-triisopropylbiphenyl;
[0066] 42mL of 2.5M sec-butyllithium was added dropwise to the reaction solution at -10°C, the dropwise reaction was completed for 1 hour, 26.7g of dicyclohexylphosphine chloride was added dropwise, and the reaction was allowed to rise naturally to room temperature for 3 hours. Add 200 mL of saturated ammonium chloride aqueous solution dropwise to the reaction liquid in an ice-water bath to quench, separate the liquid, desolventize the organic phase, add methanol to crystallize, and filter to obtain white 2-dicyclohexylphosphine-2,4,6-triiso Propyl biphenyl 42g, yield 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com