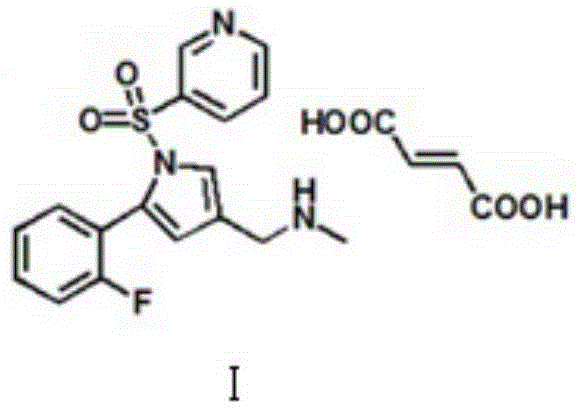
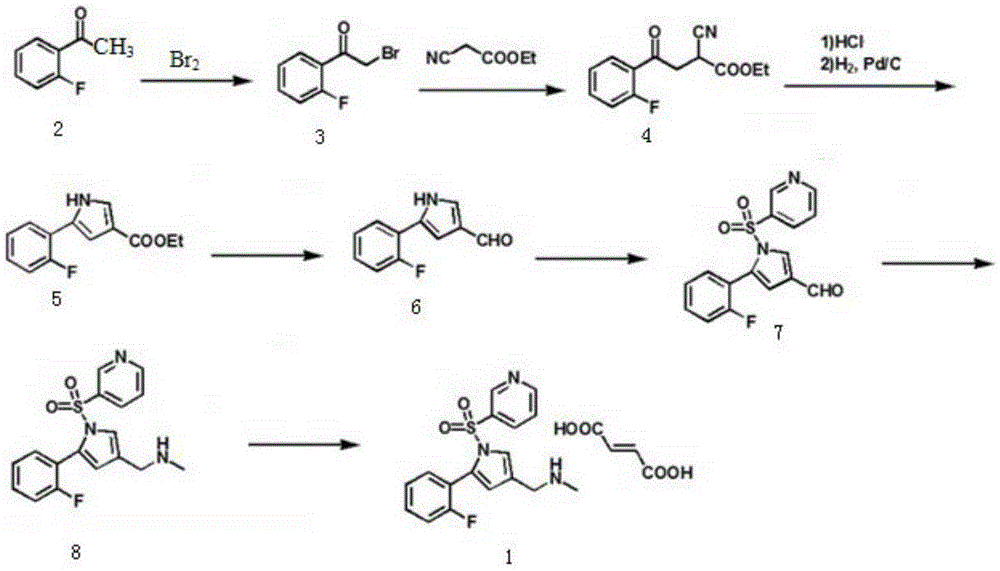
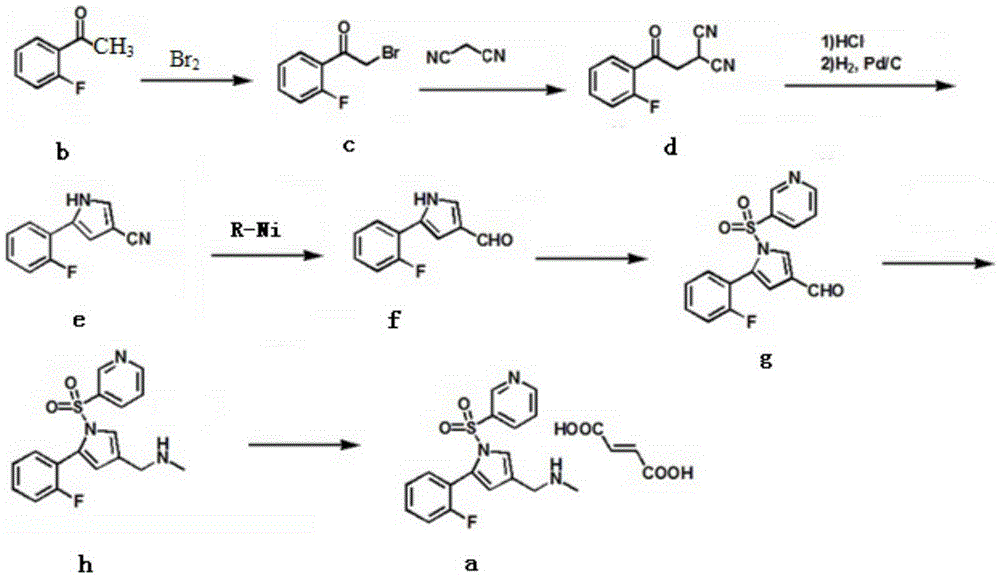
Preparation technology for vonoprazan fumarate
A technology for the preparation of vonoprazan fumarate and its preparation technology, which is applied in the field of preparation technology of vonoprazan fumarate, can solve the problems of many impurities, unsuitability for large-scale industrial production, cumbersome reaction operation steps, etc., and achieve high yield High efficiency, less impurities, and more efficient synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Preparation of o-fluorostyrylamine (Ⅲ)
[0039] 24 mL of o-fluoroacetophenone, 46.2 g of ammonium acetate, and 60 ml of ethanol were sequentially added into a 150 ml round-bottomed flask, heated to reflux, and stirred for 4 hours. After the reaction was completed, it was concentrated under reduced pressure to obtain 16.7 g of o-fluorostyrylamine (III), with a yield of 72.8% and a purity of 99.8% by HPLC.
Embodiment 2
[0040] Embodiment 2: Preparation of o-fluorostyrylamine (Ⅲ)
[0041] Into a 150ml round bottom flask, add 24mL o-fluoroacetophenone, 30.8g ammonium acetate, and 60ml ethanol in sequence, heat to reflux, stir and react for 6 hours, after the reaction, concentrate under reduced pressure to obtain 16.6g o-fluorostyrylamine (Ⅲ) , yield 72.0%, HPLC purity 99.5%.
Embodiment 3
[0042] Embodiment 3: the preparation of o-fluorostyrylamine (Ⅲ)
[0043] 24 mL of o-fluoroacetophenone, 15.4 g of ammonium acetate, and 60 ml of methanol were sequentially added into a 150 ml round-bottomed flask, heated to reflux, and stirred for 4 hours. After the reaction was completed, it was concentrated under reduced pressure to obtain 15.6 g of o-fluorostyrylamine (III), with a yield of 68% and a purity of 99.7% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com