Optimized HIL-17RA-HSA (human interleukin-17 receptor-human serum albumin) fusion gene encoding proteins
A technology of fusion gene and fusion protein, which is applied in the field of codon-optimized HIL-17RA-HSA fusion gene and its encoded protein, and can solve problems such as hindering the development prospect of cytokines and poor pharmacokinetic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H
[0025] The codon optimization of embodiment 1HIL-17RA-HSA gene
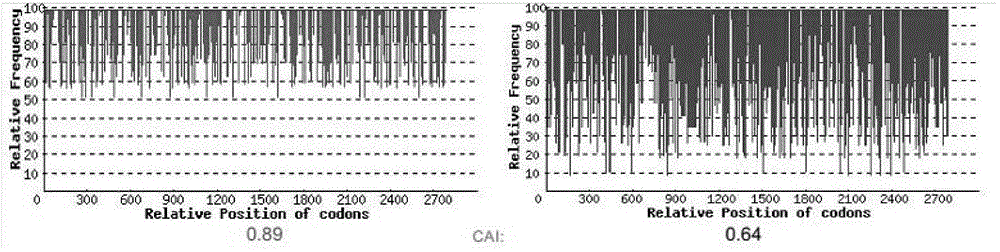
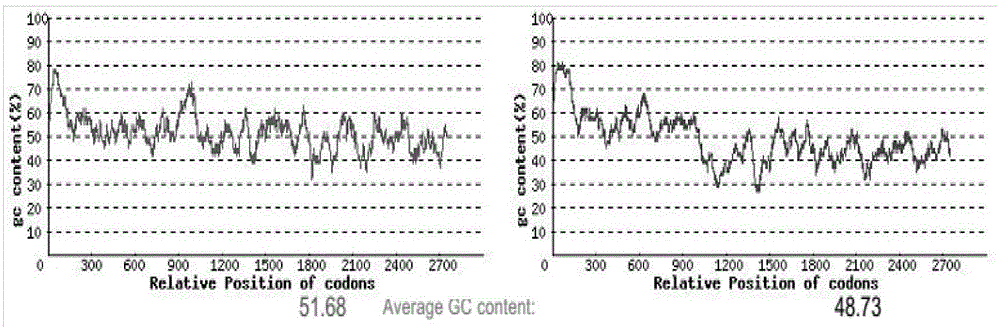
[0026] In order to achieve a higher level of expression of the HIL-17RA-HSA gene in Escherichia coli and better function, according to the codon usage preference of HIL-17RA-HSA expressed in Escherichia coli, GenScript rare codon analysis software was used The cloned HIL-17RA-HSA gene was optimally designed to increase the codon adaptation index CAI value.
[0027] Rare codons in the HIL-17RA-HSA gene will reduce the transcription level and translation efficiency in E. coli. According to the codon preference of Escherichia coli, the HIL-17RA-HSA gene was optimized, and the new sequence obtained was designated as HIL-17RA-HSA (SEQ ID NO: 1). Sequence alignment results showed that the similarity with the wild-type HIL-17RA-HSA sequence was 77.3%, and the amino acid sequence remained unchanged. Among the 2799 amino acids, the codons of 1686 amino acids were optimized, and the optimization rate reached 60%.
[002...
Embodiment 2
[0029] Example 2 Recombination. Construction of pET30a / HIL-17RA-HSA expression vector
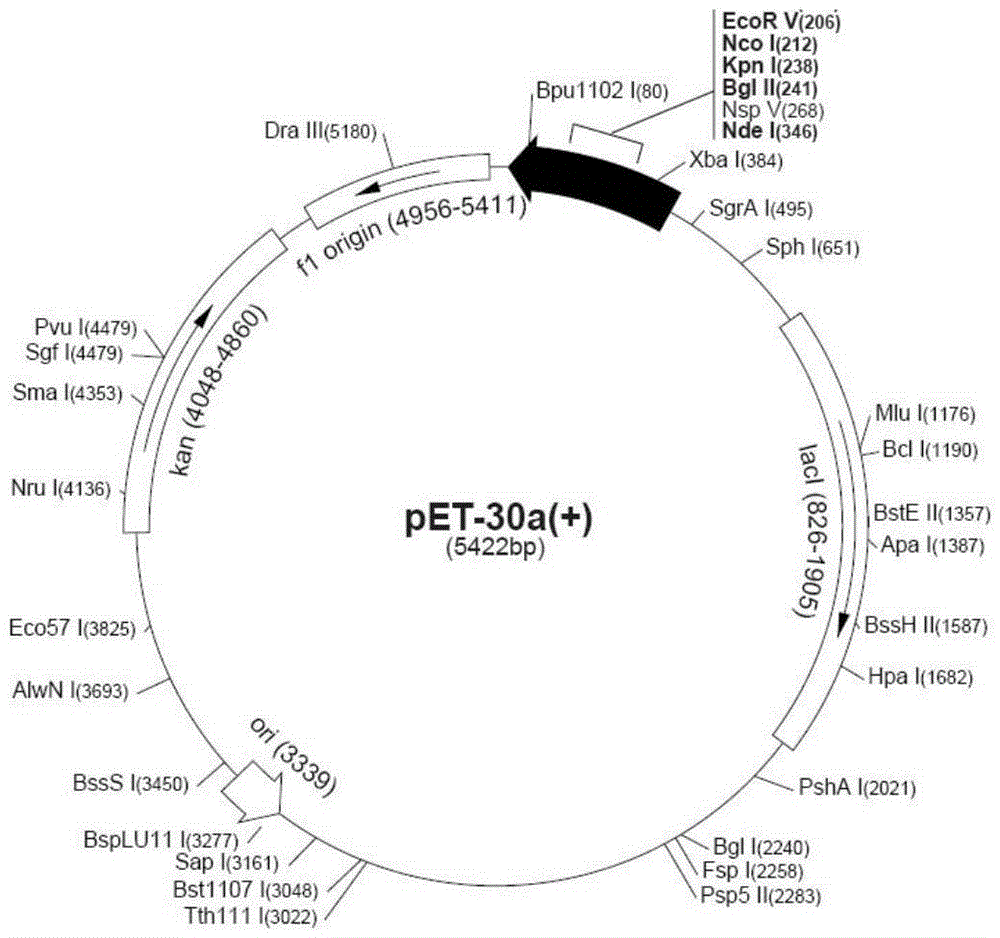
[0030] The whole gene synthesis HIL-17RA-HSA was connected into the pUC57-T vector ( image 3 ). The correct sequenced positive pUC57-T / HIL-17RA-HSA bacteria extracted plasmid (operate according to Sangong plasmid mini-extraction kit). Plasmid pET30a and pUC57-T / HIL-17RA-HSA were double digested with Xbal / HimdⅢ restriction endonuclease. The digested products were ligated, constructed into a prokaryotic expression vector pET30a / HIL-17RA-HSA, transformed into BL21(DE3) competent cells, and used Kan + The antibiotic plate was screened, and 10 single colonies were randomly selected for PCR identification with specific primers, and a bright band appeared at about 2800bp, which was consistent with the expected size ( Figure 4 ).
Embodiment 3
[0031] Example 3. Expression of recombinant plasmids in Escherichia coli
[0032] (1) Transform the recombinant expression plasmid pET30a / HIL-17RA-HSA and the empty plasmid pET30a into Escherichia coli BL21 (DE3) competent bacteria, plate and pick bacteria;
[0033] (2) Containing Kan + After shaking culture in the LB medium at 37°C overnight, take the empty vector bacterial solution and the recombinant bacteria into 3 mL and 30 mL LB medium containing kanapenicillin at a volume ratio of 1:100, respectively, and shake at 200 rpm at 37°C;
[0034] (3) When the measured OD600 value is 0.6-0.8, take out 3mL recombinant bacteria solution / tube, including the empty vector pET30a bacteria solution, and add 1mMIPTG, continue to culture at 15°C and 37°C for 4-16h, and induce for 4h, respectively. Take 1 tube each at 16 hours. In addition, take 1 mL of the bacterial liquid that has not been induced by IPTG as a control;
[0035] (4) Centrifuge the expressed bacterial solution at 6500...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com