Polyceptor-structure small molecule compound and preparing method and application thereof
A technology of small molecular compounds and compounds, applied in semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc., can solve the problems of small-molecule photovoltaic materials that are rarely reported, and achieve improved planar configuration and high photoelectric conversion efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
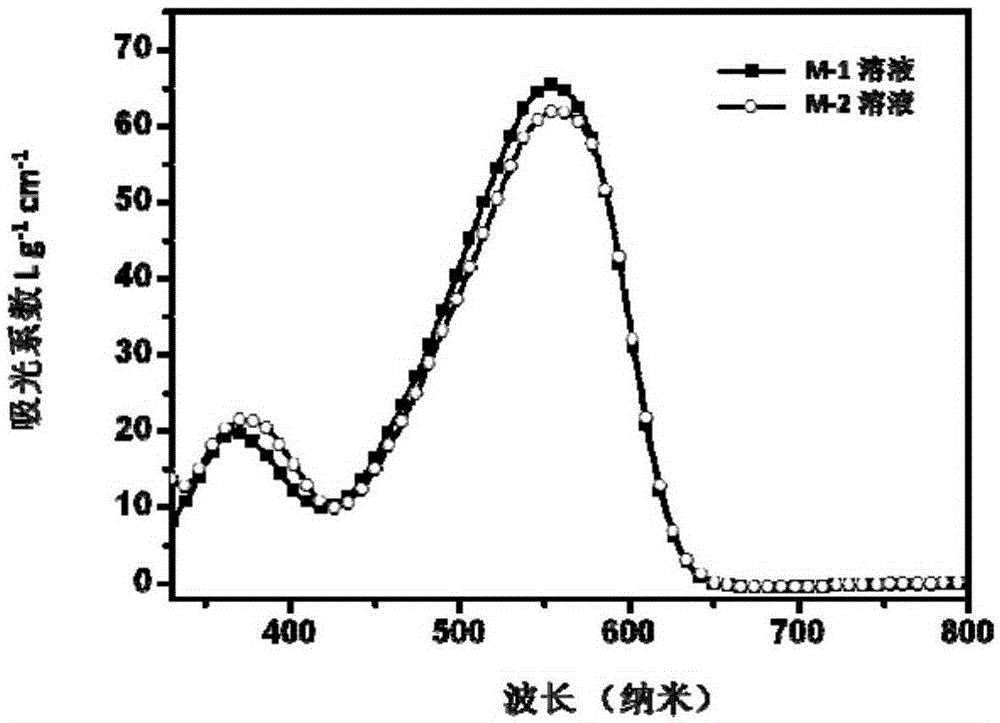
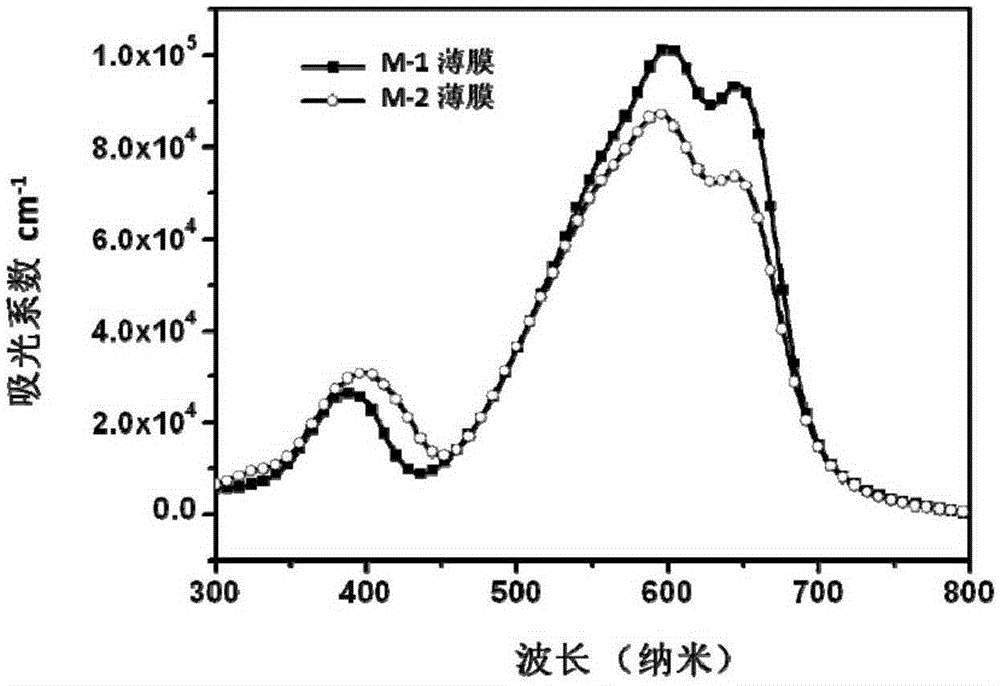
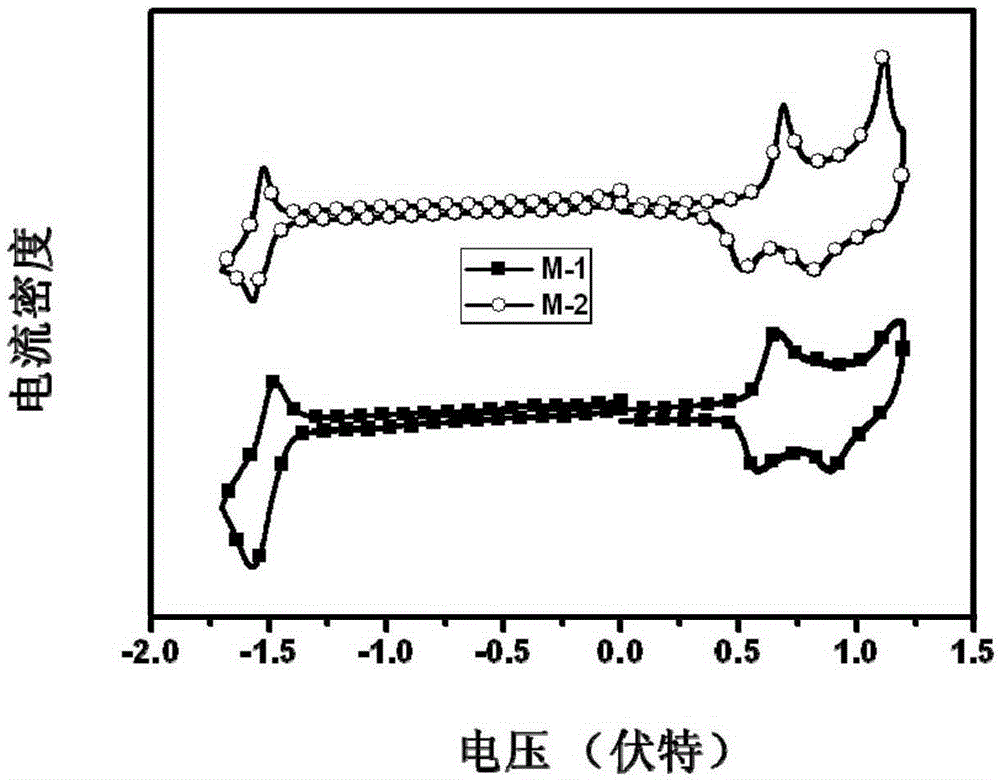
[0057] Embodiment 1 provides the synthesis of a photovoltaic multi-acceptor structure small molecule compound with an M-1 structure,
[0058]
[0059] Concrete synthetic route is:
[0060]
[0061] The specific steps are:
[0062] (1) (5-(2,5-bis((2-butyloctyl)oxy)-4-(thiophen-2-yl)phenyl)thiophen-2-yl)trimethyltin (compound 2 )Synthesis:
[0063] Add 2,2'-(2,5-bis((2-butyloctyl)oxy)-1,4-phenylene)dithiophene (compound 1) (1.53g, 2.5 mmol) and 40mL of anhydrous tetrahydrofuran were stirred and dissolved; the solution was cooled to -78°C in a nitrogen atmosphere, and a n-hexane solution of n-butyllithium (2.4M, 1.04mL, 2.5mmol) was slowly added dropwise, and reacted at this temperature for 1 Hours; then rise to room temperature and react for 1 hour; cool to -78°C, add trimethyltin chloride in tetrahydrofuran solution (1.0M, 2.5mL, 2.5mmol) in one go, react for 1 hour; rise to room temperature and react for 3 hours After that, pour into water to quench;
[0064] After...
Embodiment 2
[0085] Embodiment 2 provides the synthesis of a photovoltaic multi-acceptor structure small molecule compound with M-2 structure,
[0086]
[0087] Concrete synthetic route is:
[0088]
[0089] The specific steps are:
[0090] (1) Synthesize (5-(2,5-bis((2-butyloctyl)oxy)-4-(thiophen-2-yl)phenyl)thiophene-2- according to Example 1 step (1) base) trimethyltin (compound 2)
[0091] (2) 4-(5-(2,5-bis((2-butyloctyl)oxy)-4-(thiophen-2-yl)phenyl)thiophen-2-yl)-7-bromo- Synthesis of 5,6-fluorobenzene[c][1,2,5]thiadiazole (Compound 5):
[0092] Add compound 2 (3.87g, 5mmol), 4,7-dibromo-5,6-difluorobenzo[c][1,2,5]thiadiazole (1.65g, 5mmol) into a dry 250mL three-necked flask , 120mL THF, and Pd(PPh 3 ) Cl 2 (105mg, 0.15mmol); Stir to dissolve, use a vacuum pump to pump nitrogen gas 3 times; heat up to 70°C for 24 hours; cool to room temperature and spin evaporate directly, and rinse with dichloromethane:petroleum ether (1:5) Pass through a silica gel column to obtain an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical band gap | aaaaa | aaaaa |
| optical band gap | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com