Diphenylamine organic neutral free radical electroluminescent material as well as preparation and application thereof
An electroluminescent material, diphenylamine technology, applied in the direction of luminescent material, preparation of organic compounds, preparation of amino compounds, etc., can solve the problems of few types of free radicals, harsh reaction conditions, complicated synthesis steps, etc., to improve the stability , the effect of increasing the degree of conjugation and reducing the electron density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of compound 1:
[0046]
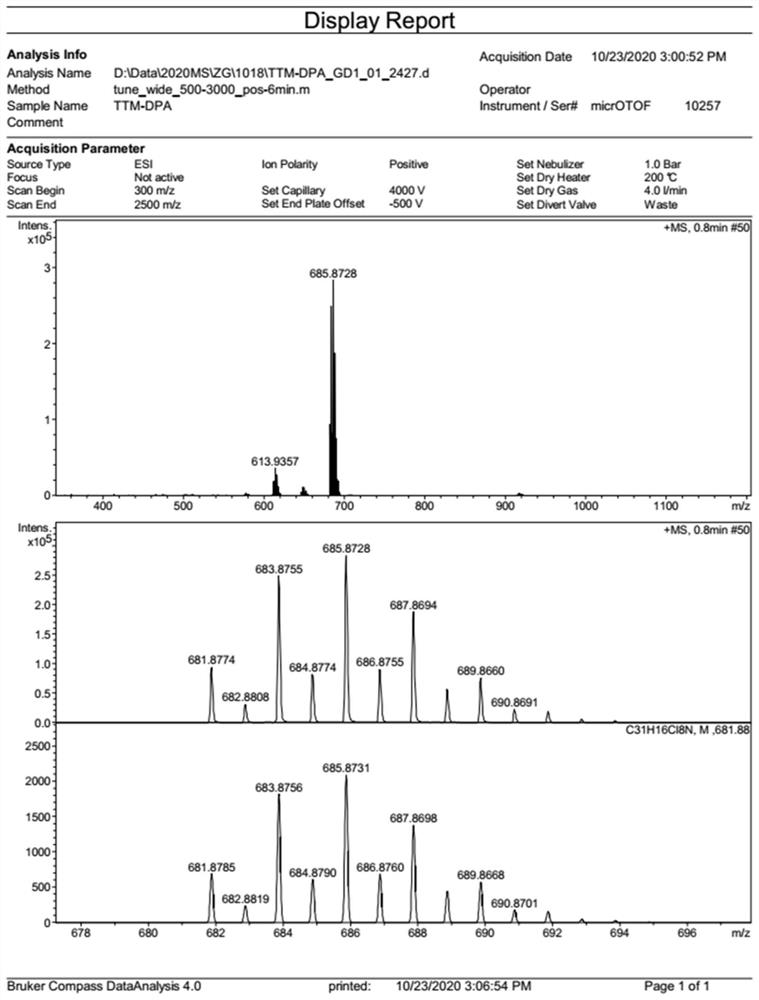
[0047] Under nitrogen atmosphere, weigh tris(2,4,6-trichlorophenyl)methyl radical (830mg, 1.5mmol), diphenylamine (169mg, 1.0mmol), Pd(OAc) 2 (74mg, 0.33mmol), P(t-Bu) 3 (2.0g, 10wt% toluene solution, 0.99mmol) and Cs 2 CO 3 (1.47g, 4.5mmol), dissolved in 20mL of toluene, heated to 80°C, and reacted for 16 hours. After the reaction was finished, the toluene in the reaction system was removed, and the crude product was separated and purified by silica gel column chromatography to obtain 515 mg of compound 1 with a yield of 75%. Its mass spectrum and single crystal structure were shown in figure 1 , Figure 5 shown. Since the free radical has no NMR signal, it is characterized by high-resolution mass spectrometry, and the product HRMS (ESI, m / z): calculated value, 685.8732; measured value, 685.8728.
Embodiment 2
[0049] Preparation of Compound 2:
[0050]
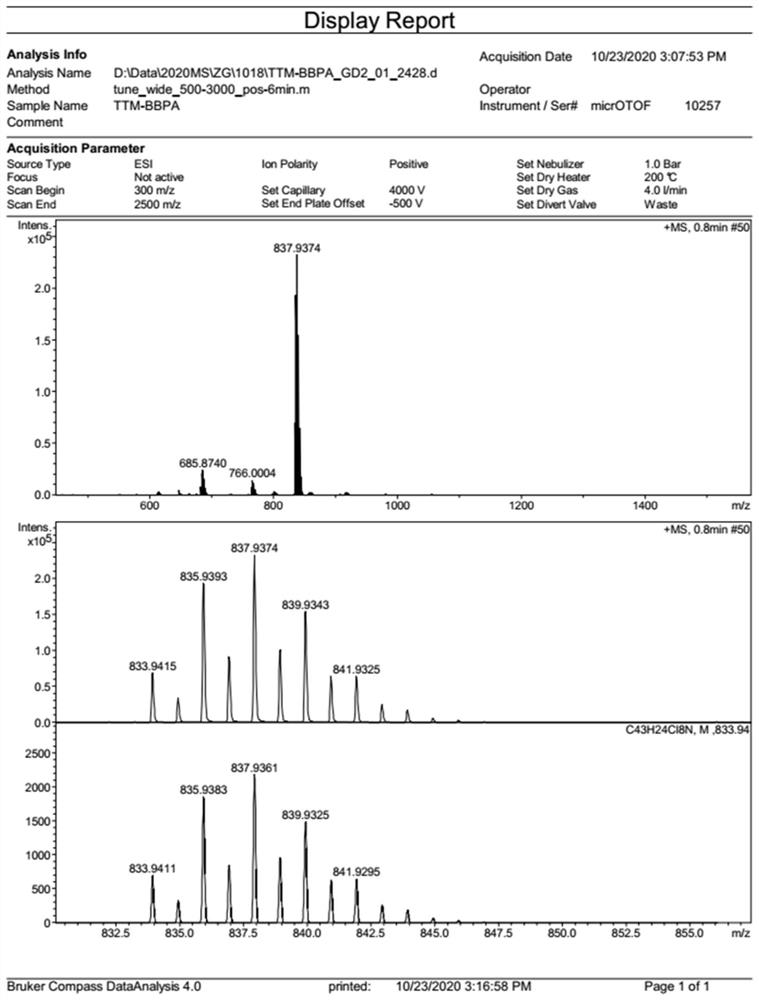
[0051] Under nitrogen atmosphere, weigh tris(2,4,6-trichlorophenyl)methyl radical (830mg, 1.5mmol), diphenylamine (643mg, 3.8mmol), Pd(OAc) 2 (74mg, 0.33mmol), P(t-Bu) 3 (2.0g, 10wt% toluene solution, 0.99mmol) and Cs 2 CO 3 (1.47g, 4.5mmol), dissolved in 20mL of toluene, heated to 100°C, and reacted for 20 hours. After the reaction, the toluene in the reaction system was removed, and the crude product was separated and purified by silica gel column chromatography to obtain 762 mg of compound 2 with a yield of 62%. Since the free radical has no NMR signal, it is characterized by high-resolution mass spectrometry, and the product HRMS (ESI, m / z): calculated value, 816.9886; measured value, 816.9875.
Embodiment 3
[0053] Preparation of compound 3:
[0054]
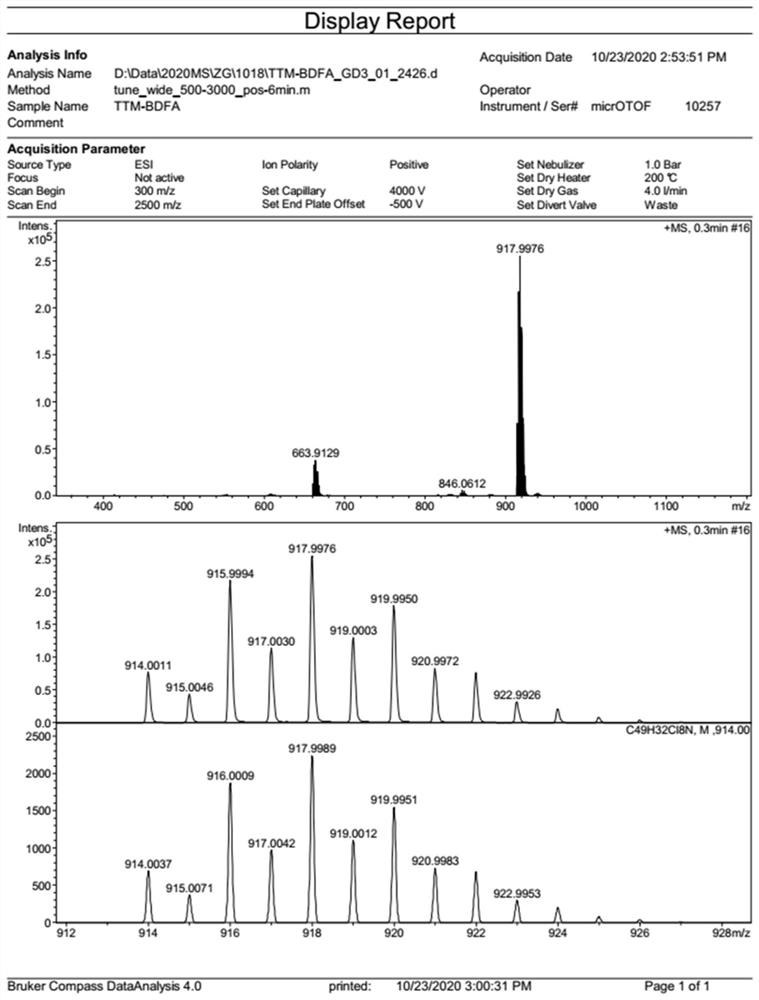
[0055] Under nitrogen atmosphere, weigh tris(2,4,6-trichlorophenyl)methyl radical (830mg, 1.5mmol), diphenylamine (896mg, 5.3mmol), Pd(OAc) 2 (74mg, 0.33mmol), P(t-Bu) 3 (2.0g, 10wt% toluene solution, 0.99mmol) and Cs 2 CO 3 (1.47g, 4.5mmol), dissolved in 20mL of toluene, heated to 100°C, and reacted for 36 hours. After the reaction, the toluene in the reaction system was removed, and the crude product was separated and purified by silica gel column chromatography to obtain 856 mg of compound 3 with a yield of 60%. Since the free radical has no nuclear magnetic signal, it is characterized by high-resolution mass spectrometry, and the product HRMS (ESI, m / z): calculated value, 950.1011; measured value, 950.1019.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com