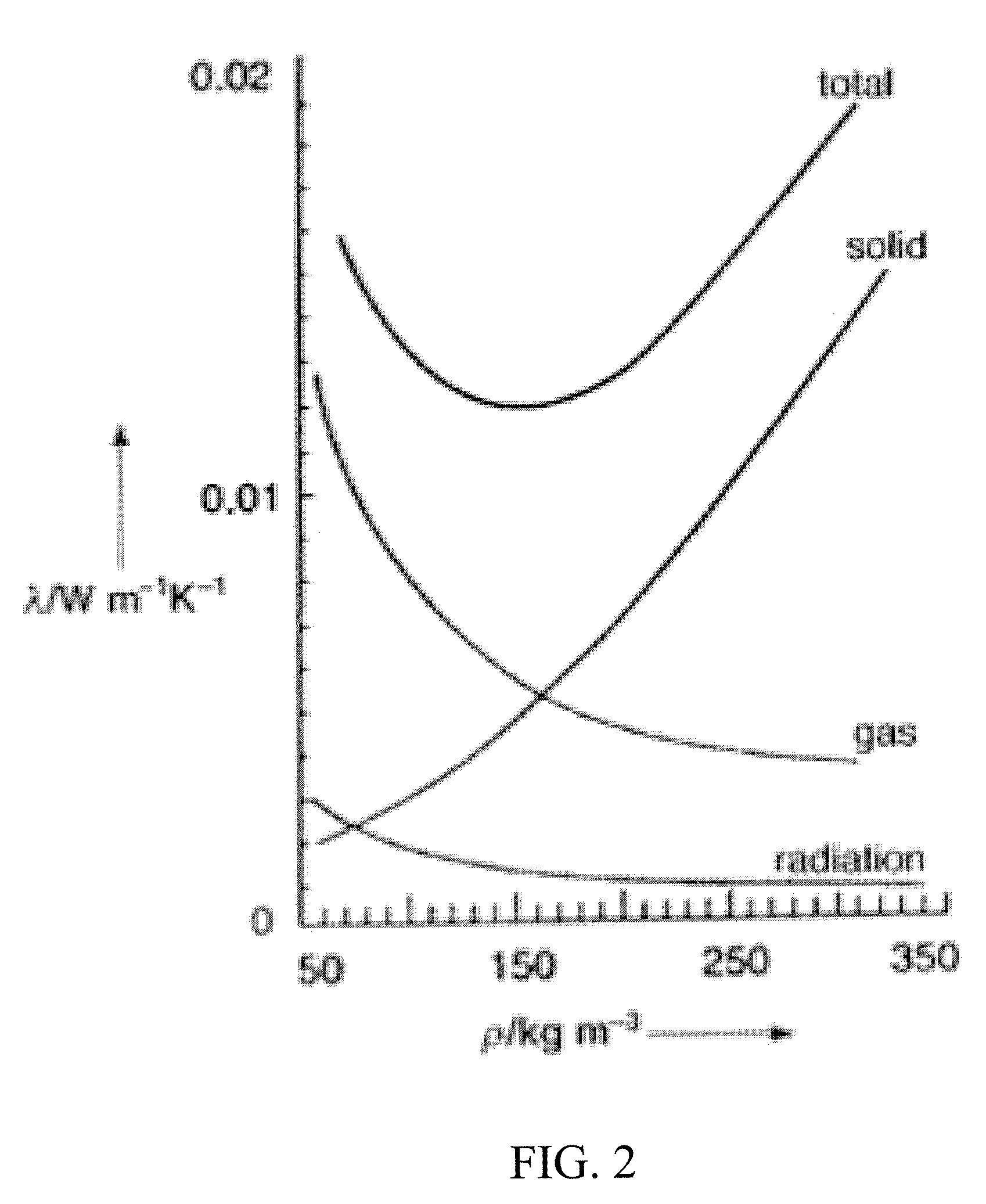Patents
Literature
59results about How to "Reduce electron density" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
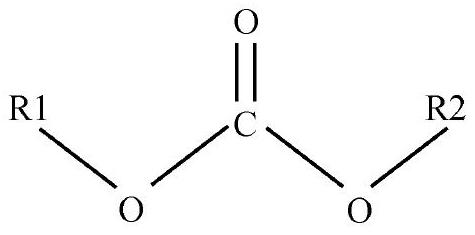
Iridium Complex Containing Carbazole-Substituted Pyridine and Phenyl Derivatives as Main Ligand and Organic Light-Emitting Diodes Containing the Same
ActiveUS20100270540A1Reduce electron densityIncrease electron densityGroup 8/9/10/18 element organic compoundsElectroluminescent light sourcesIridiumDopant
The present invention relates to a novel iridium complex into which carbazole-substituted pyridine derivatives and various substituents-substituted phenyl derivatives are introduced as main ligand and a electrophosphorescence diode containing the same as a dopant of a light-emitting layer. When the iridium complex according to the present invention is applied to an organic light-emitting diode, the heat-resistance property and the light-emitting property can be significantly improved as well as the light-emitting efficiency and the like can be significantly improved by doping the iridium complex compound into the light-emitting layer as compared to the conventional organic light-emitting diode.
Owner:INKTEC CO LTD +1
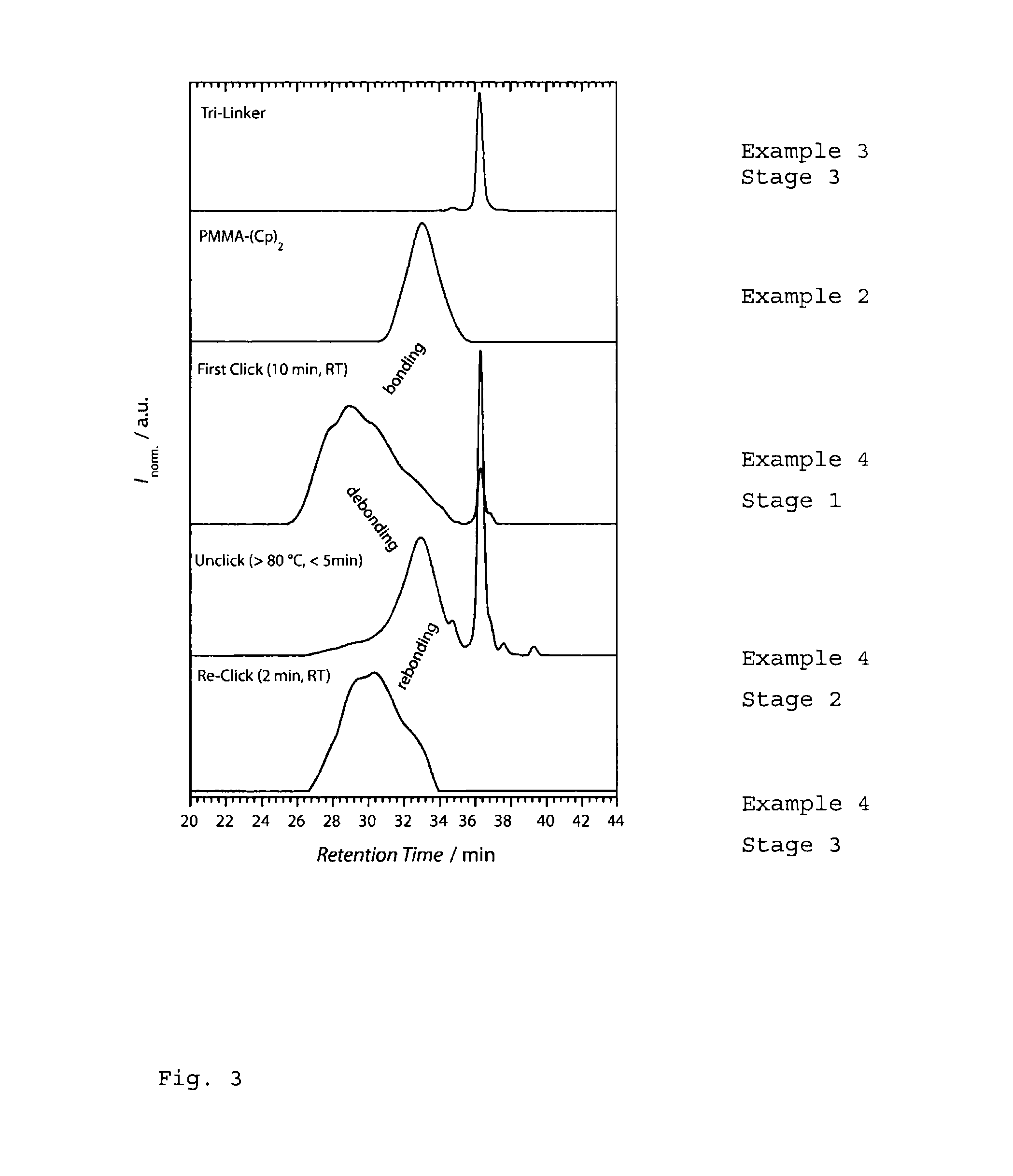
Functional materials with reversible crosslinking
InactiveUS20120309895A1Quick activationReduce electron densityAdhesive processesInksAdhesiveRoom temperature
The present invention relates to an innovative method for the reversible crosslinking of, for example, adhesives or coating materials. The reversible crosslinking method allows very rapid crosslinking even at room temperature and undoing of the crosslinks at higher temperatures, thereby regaining the capacity for thermoplastic processing and allowing the originally bonded substrates to be separated from one another again with ease. A particular aspect in this context is that a plurality of cycles of crosslinking and undoing of the crosslinks are possible with the present system.
Owner:EVONIK OPERATIONS GMBH
Silica aerogels and their preparation
ActiveUS20120128958A1Promote formationGreater tenabilitySilicaAbsorbent padsFlexural modulusRefractive index
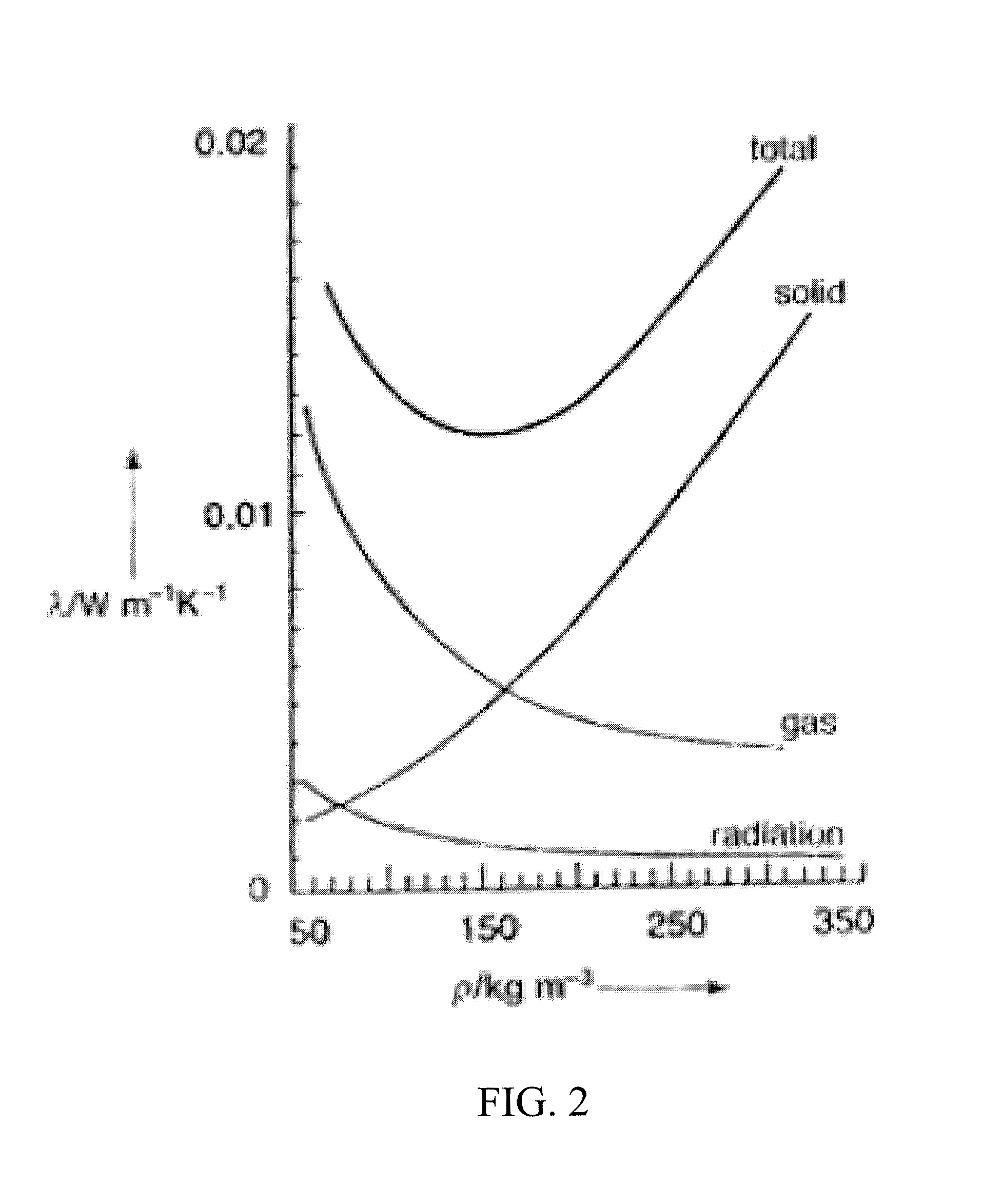
Silica aerogels with improved properties are disclosed together with methods for synthesizing such aerogels. The improved properties include lower thermal conductivity (better insulating capacity), lower acoustic velocity, lower dielectric constant and improved ductility. Greater tunability of the refractive index can also be achieved. The silica aerogels are prepared by a sol-gel processing method that provides better control of the formation of aerogel structures. Generally speaking, the improvements arise from control of the synthesis to create a morphology of primary clusters and diverse-sized secondary clusters of dense silica aerogels separated by less densely packed regions. By providing a broader range of secondary clusters and / or pore sizes and loose connectivity between clusters, reductions can be achieved in thermal conductivity and flexural modulus.
Owner:MASSACHUSETTS INST OF TECH
Silicon-containing polymer, resist composition and patterning process
InactiveUS6902772B2High sensitivityImprove heat resistancePhotosensitive materialsPhotomechanical apparatusResistAryl
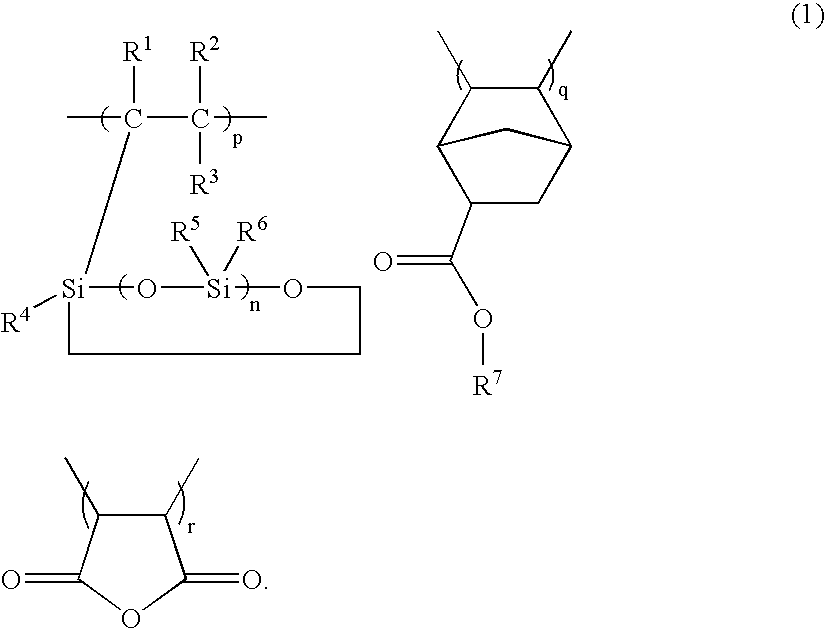
Silicon-containing polymers comprising recurring units of three components represented by the general formula (1) are novel wherein R1, R2 and R3 are hydrogen or C1-10 alkyl, R4, R5 and R6 are hydrogen, C1-20 alkyl or haloalkyl, or C6-20 aryl, R7 is C4-20 alkyl, n is 1 to 5, p, q and r are positive numbers. Resist compositions comprising the polymers are sensitive to high-energy radiation and have a high sensitivity and resolution at a wavelength of less than 300 nm and improved resistance to oxygen plasma etching
Owner:SHIN ETSU CHEM IND CO LTD
Electrolyte comprising eutectic mixture and secondary battery using the same
ActiveUS20100239917A1Reduce electron densityReduce compoundingAlkaline accumulatorsNon-aqueous electrolyte cellsLithiumElectrochemistry
Disclosed is an electrolyte for a secondary battery, comprising an eutectic mixture consisting of: (a) am amide group-containing compound with at least one EDG introduced into the N-position thereof; and (b) an ionizable lithium salt. Also, provided are a secondary battery comprising such an electrolyte, and a method of adjusting an electrochemical stability window of an eutectic mixture consisting of an amide group-containing compound and a lithium salt by regulating electron donating properties of at least one substituent group introduced into the N-position of the amide group-containing compound.
Owner:LG ENERGY SOLUTION LTD
Silica aerogels and their preparation
InactiveUS9073759B2Greater tenabilityLow thermal conductivitySilicaLayered productsFlexural modulusRefractive index
Silica aerogels with improved properties are disclosed together with methods for synthesizing such aerogels. The improved properties include lower thermal conductivity (better insulating capacity), lower acoustic velocity, lower dielectric constant and improved ductility. Greater tunability of the refractive index can also be achieved. The silica aerogels are prepared by a sol-gel processing method that provides better control of the formation of aerogel structures. Generally speaking, the improvements arise from control of the synthesis to create a morphology of primary clusters and diverse-sized secondary clusters of dense silica aerogels separated by less densely packed regions. By providing a broader range of secondary clusters and / or pore sizes and loose connectivity between clusters, reductions can be achieved in thermal conductivity and flexural modulus.
Owner:MASSACHUSETTS INST OF TECH
Functional materials with controllable viscosity
ActiveUS20120289657A1Quick activationReduce electron densityInksOrganic non-macromolecular adhesiveChemistryAdhesive
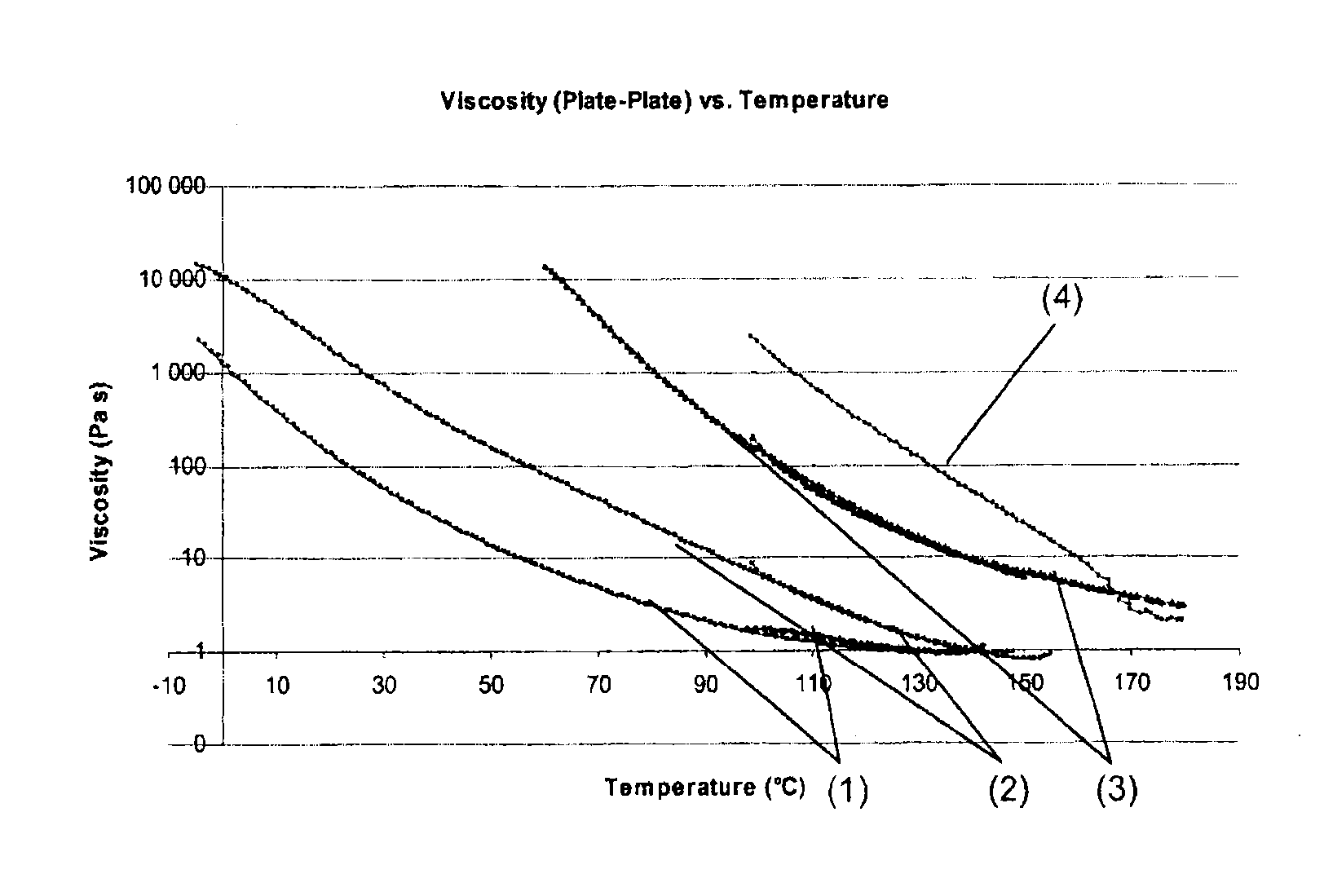
The present invention relates to an innovative method for controlling the viscosity of, for example, adhesives or coating formulations. The method for controlling viscosity allows very rapid thermoplastic curing of a formulation even at room temperature and a significant reduction in the viscosity at higher temperatures, thereby regaining the capacity for simple processing and allowing, for example, the originally bonded substrates to be separated from one another again with ease. A particular aspect in this context is that a plurality of cycles of thermoplastic curing and a significant reduction in the viscosity are possible with the present system.
Owner:EVONIK ROEHM GMBH
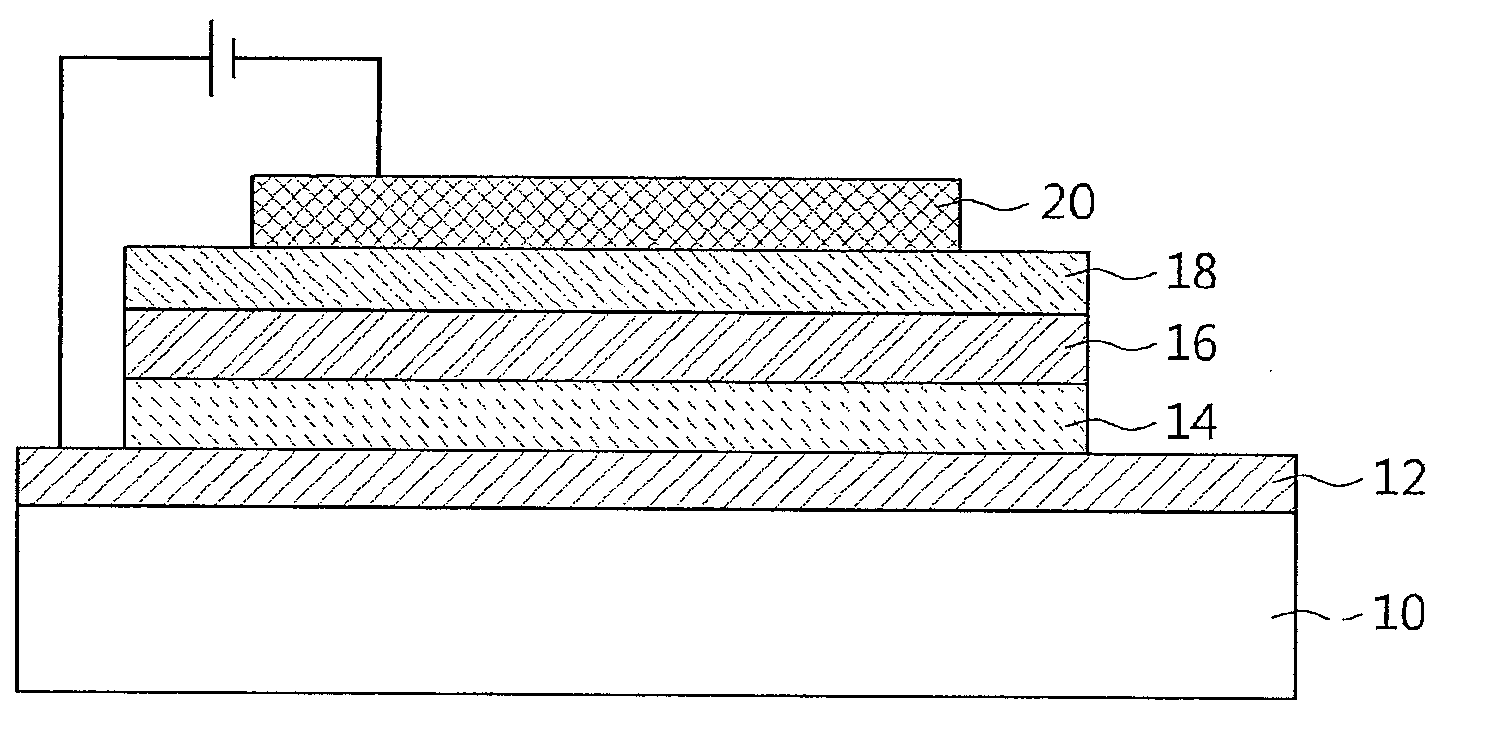
Luminescent organometallic compound and light emitting device
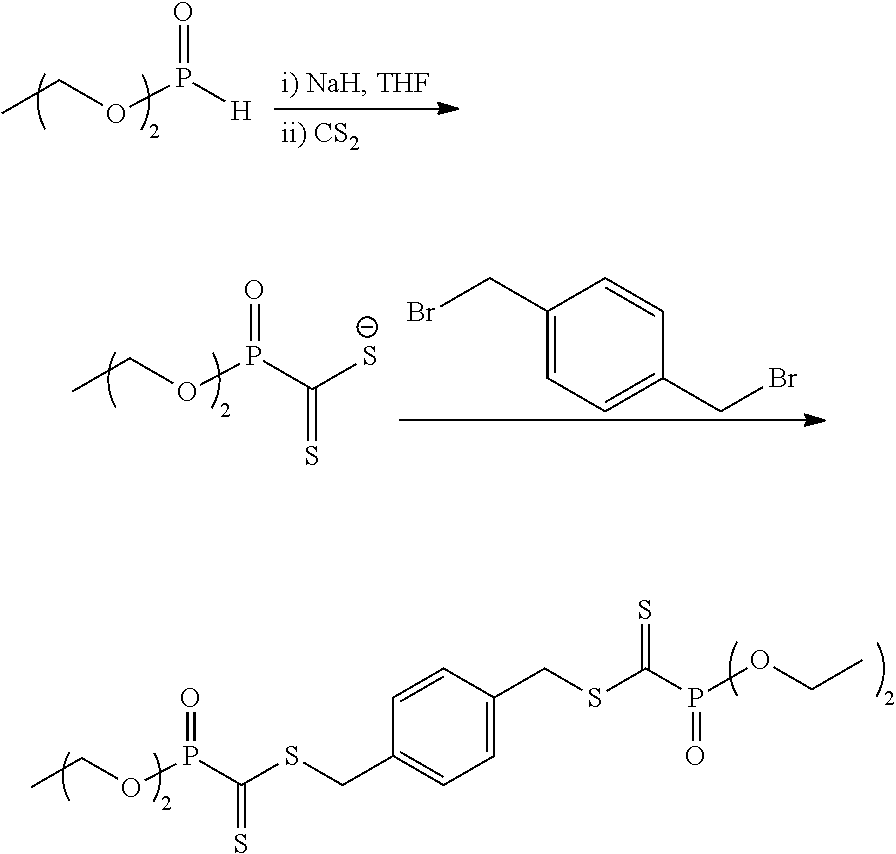
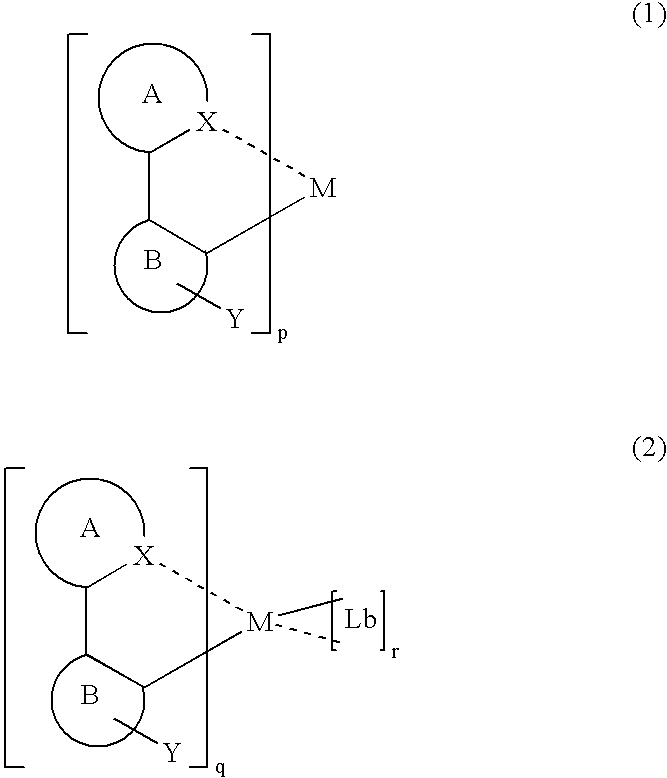
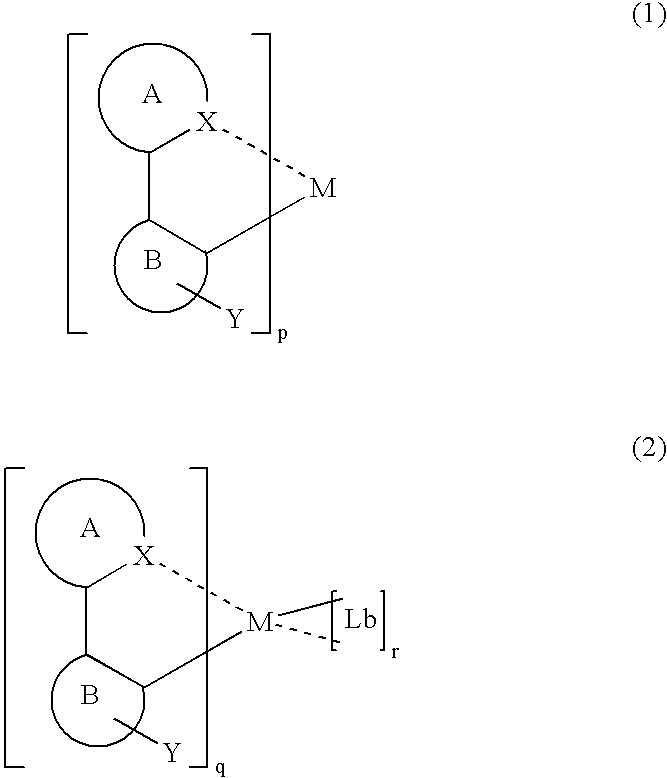
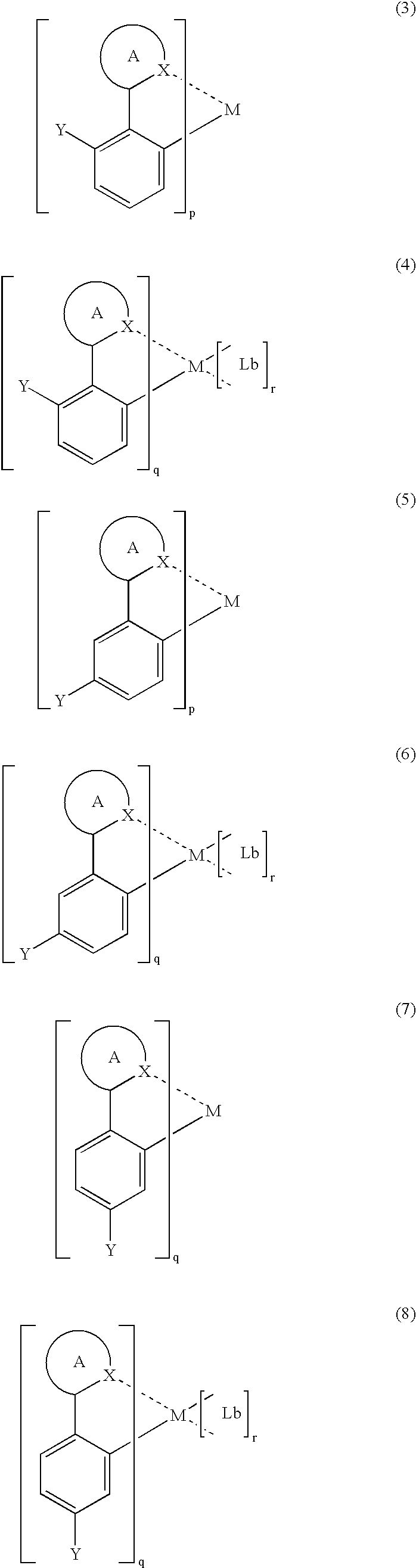
InactiveUS7067202B2Improve luminous efficiencyGood chemical stabilityIndium organic compoundsDischarge tube luminescnet screensChemical structureHydrogen
A luminescent organometallic compound having the chemical structure represented by the general formula (1) or (2):[in the general formulas (1) and (2), A and B represent ring structures, M represents a metallic atom, X represents a hetero atom other than carbon and hydrogen, Y represents at least one electron-attracting group connecting to ring structure B, Lb represents a unidentate or multidentate ligand, and p, q and r represent positive integers.]
Owner:SANYO ELECTRIC CO LTD
Pseudothermoplastic, self-crosslinking composites
InactiveUS20140323001A1Reduce electron densityRapid responseLayered productsPretreated surfacesRoom temperatureDiels alder
In the present process, reversibly crosslinking composites or storage-stable prepregs are produced by means of a hetero Diels-Alder reactions (HDA,) for example of PMMA polymers. At slightly elevated temperature, these prepregs can be reversibly decrosslinked again by a retro hetero Diels-Alder reaction so that they become moldable. The back-reaction to products which are again crosslinked or high molecular weight then takes place at room temperature.
Owner:EVONIK DEGUSSA GMBH
Materials having a controllable degree of crosslinking
ActiveUS20130303678A1Increase viscosityReduce electron densitySpecial tyresInksChemistryEnergy source
The present invention relates to innovative materials which can be crosslinked by means of two different crosslinking mechanisms, the first crosslinking mechanism being an irreversible crosslinking. The second crosslinking mechanism is a thermoreversible mechanism.As a result of this thermoreversible change in the arc length, properties of the crosslinked material can be decisively changed and controlled, including flexibility, elasticity and other mechanical properties, but also chemical properties, the gas permeability and vapour permeability and storage capacity of the network. In this way it would be possible, for example, to store energy sources such as fuels.
Owner:EVONIK ROEHM GMBH
Non-aqueous electrolyte secondary battery
ActiveUS20060166091A1Large capacitySatisfactory characteristicOrganic electrolyte cellsSecondary cellsSolventTungsten
To provide a high-capacity non-aqueous electrolyte secondary battery that exhibits satisfactory charge / discharge cycle characteristics even in a high temperature environment. The battery has: a positive electrode including a nickel-containing lithium composite oxide; a negative electrode capable of charging and discharging; a separator interposed between the positive and negative electrodes; and a non-aqueous electrolyte containing a non-aqueous solvent and a solute dissolved therein. The non-aqueous electrolyte contains a fluorine atom-containing aromatic compound. The nickel-containing lithium composite oxide is represented by, for example, LiNixM1-x-yLyO2 where element M is at least one selected from the group consisting of Co and Mn; element L is at least one selected from the group consisting of Al, Sr, Y, Zr, Ta, Mg, Ti, Zn, B, Ca, Cr, Si, Ga, Sn, P, V, Sb, Nb, Mo, W and Fe; and x and y satisfy 0.1≦x≦1 and 0≦y≦0.1.
Owner:PANASONIC CORP
Thermionic flat electron emitter
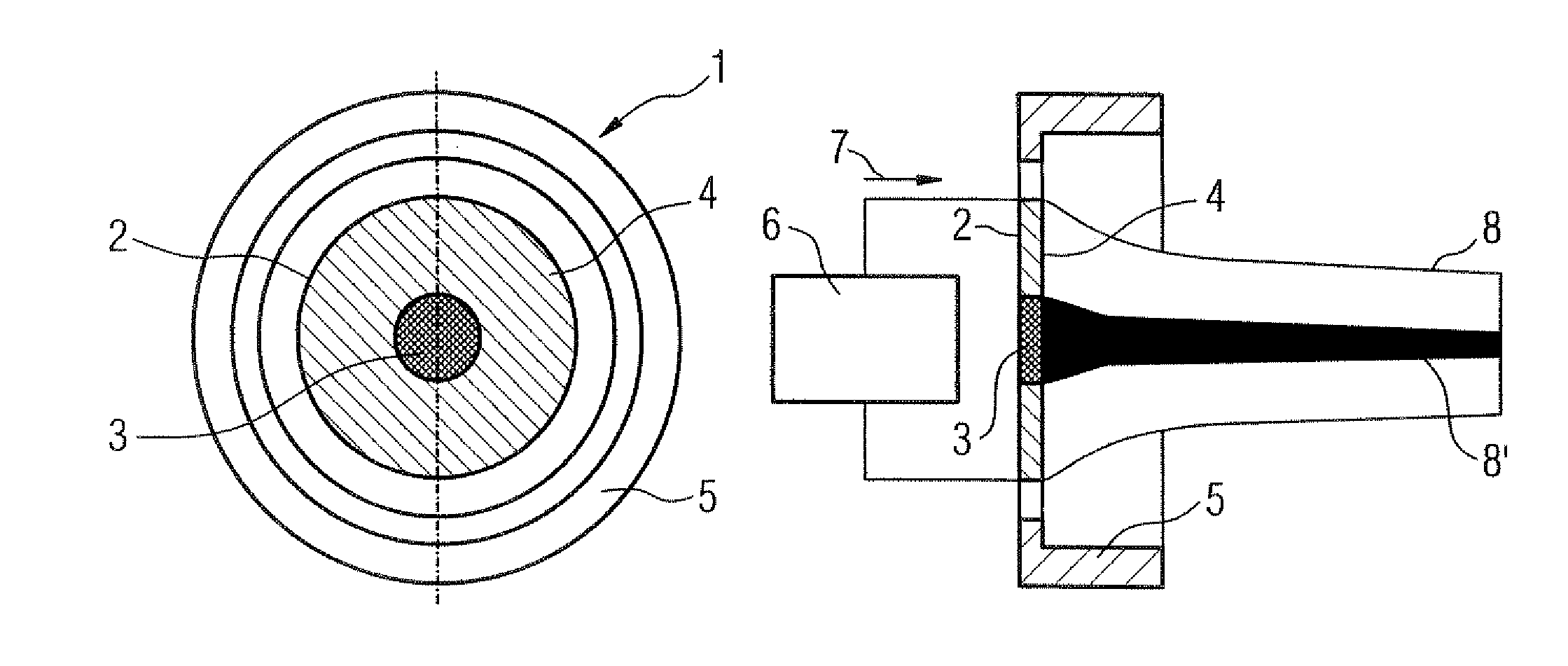
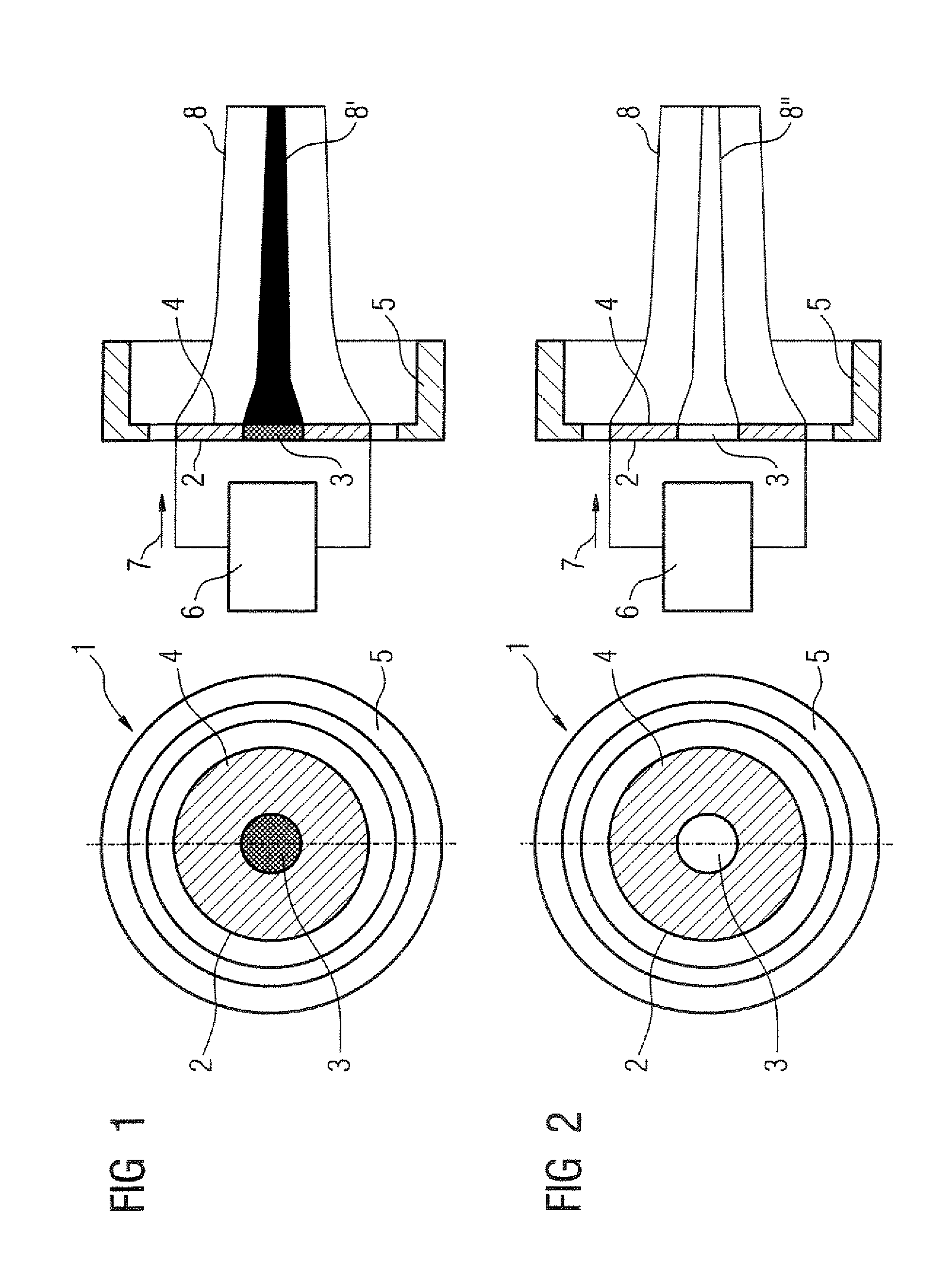
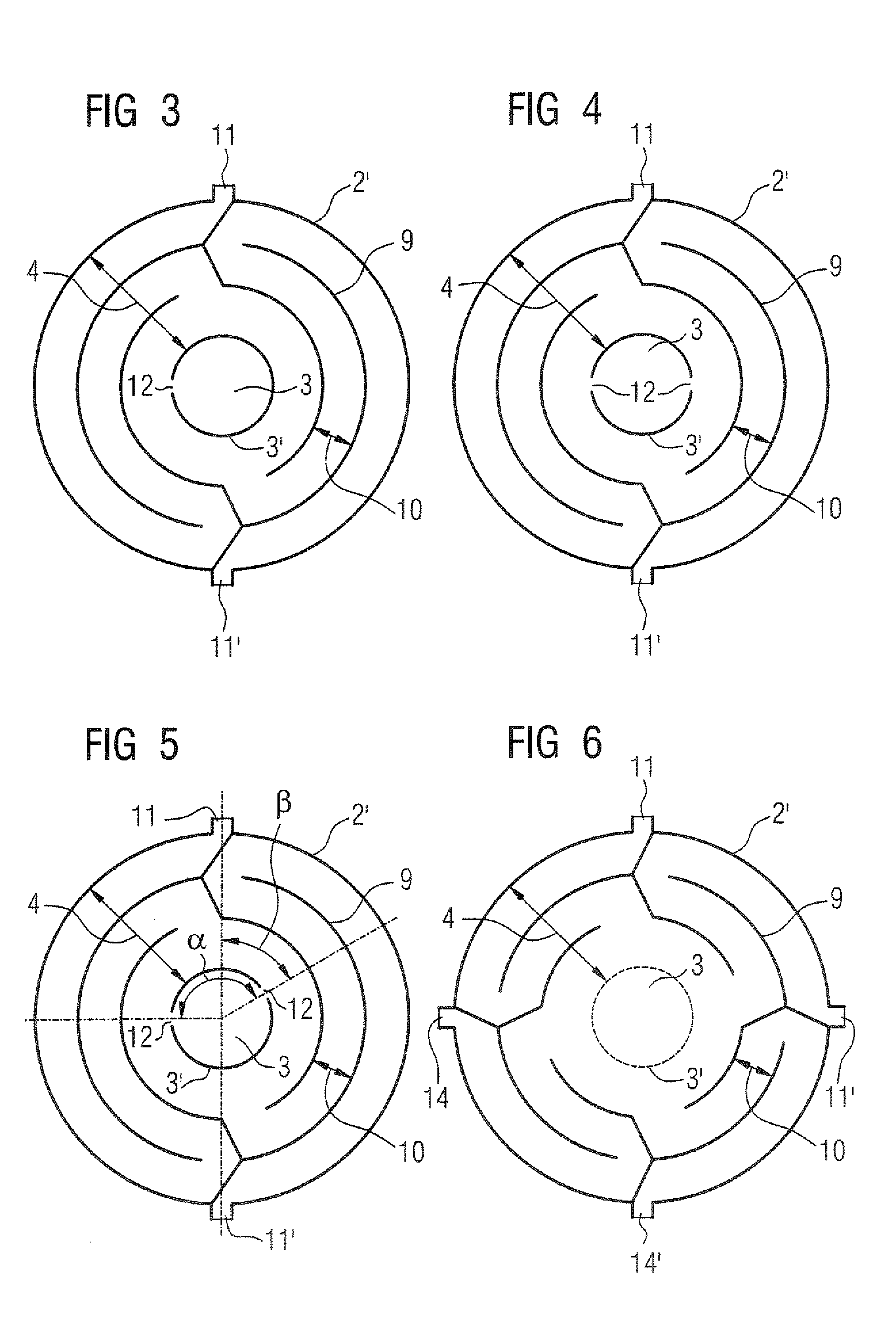
InactiveUS20070246789A1Low costProtection from damageX-ray tube electrodesSolid-state devicesElectron densityAtomic physics
A thermionic flat electron emitter has an emitter arrangement with an emitter plate having slits therein that produce serpentine current paths. The emitter arrangement has a structure that, in operation, causes the electron density of the emitted electrons to be lower in the central region of the emitter plate than in a region adjoining the central region.
Owner:SIEMENS AG
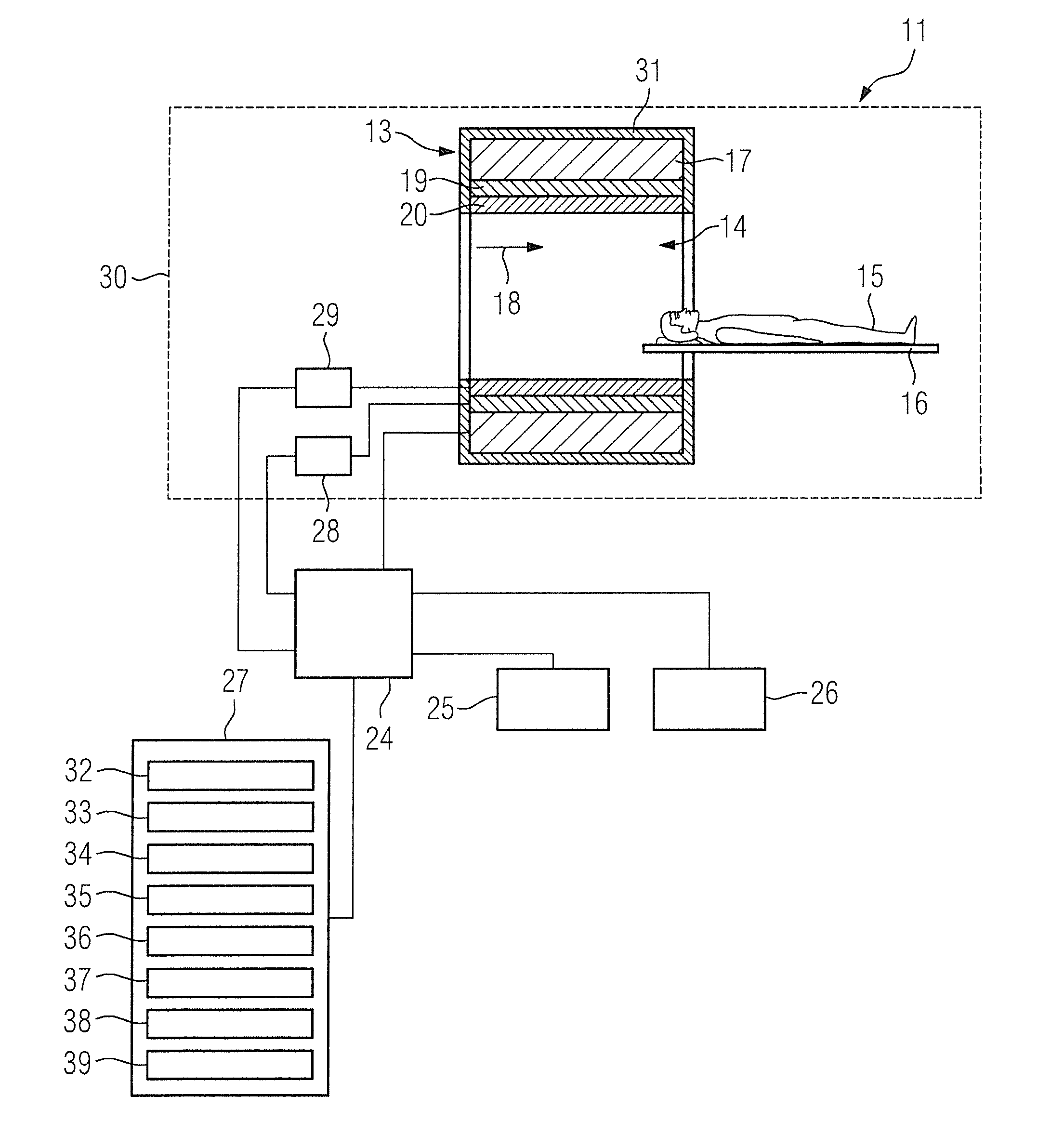
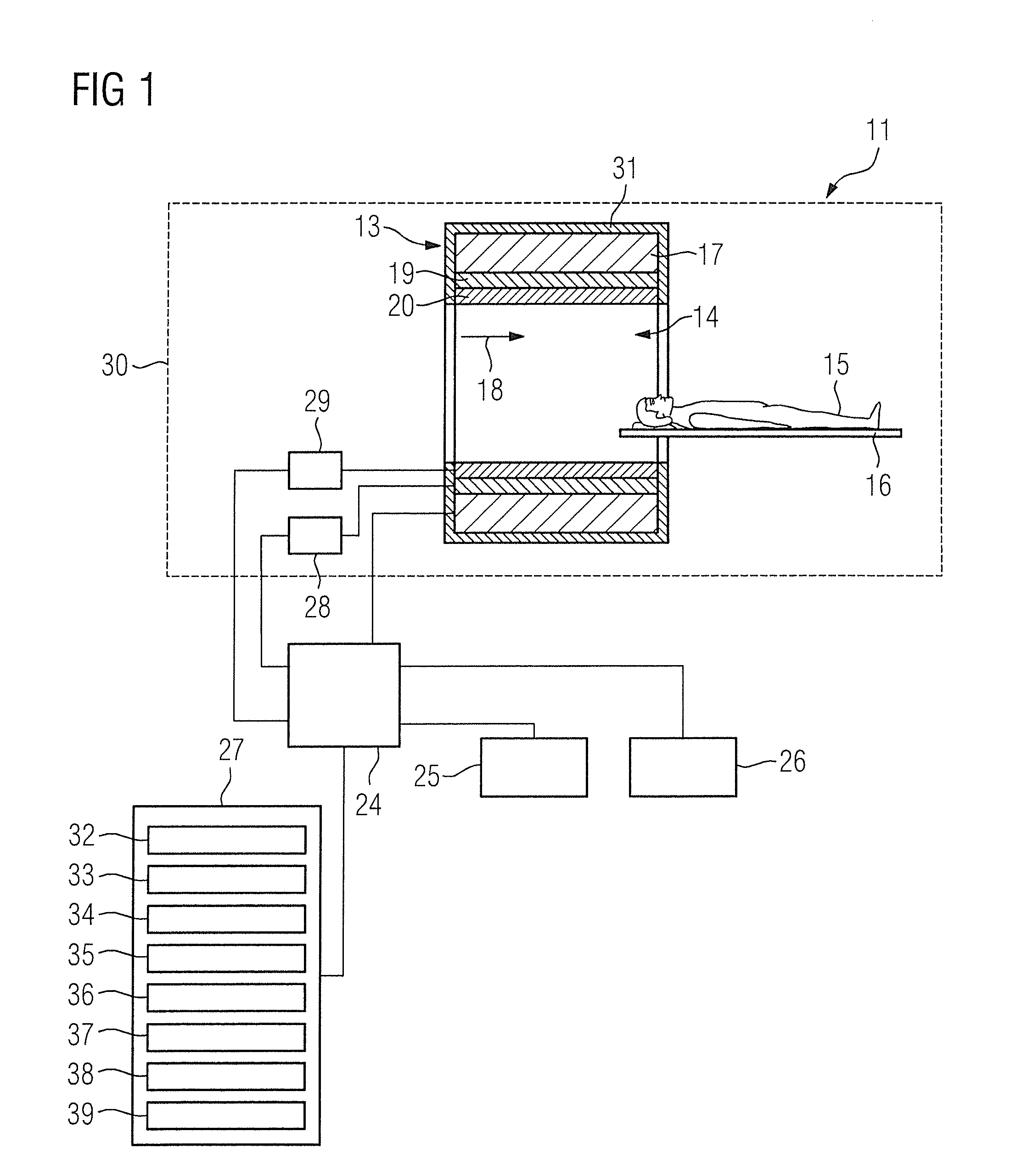
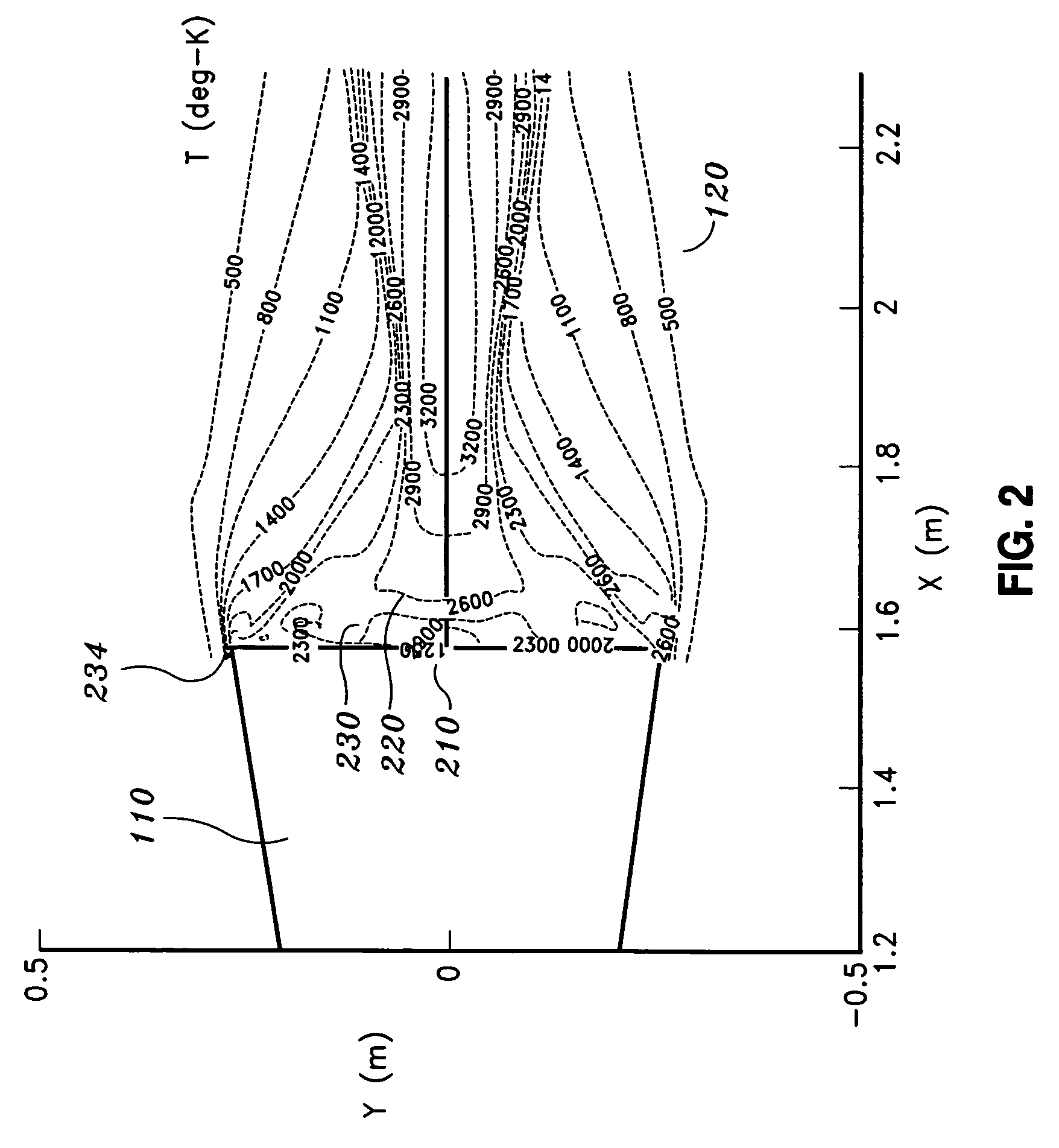
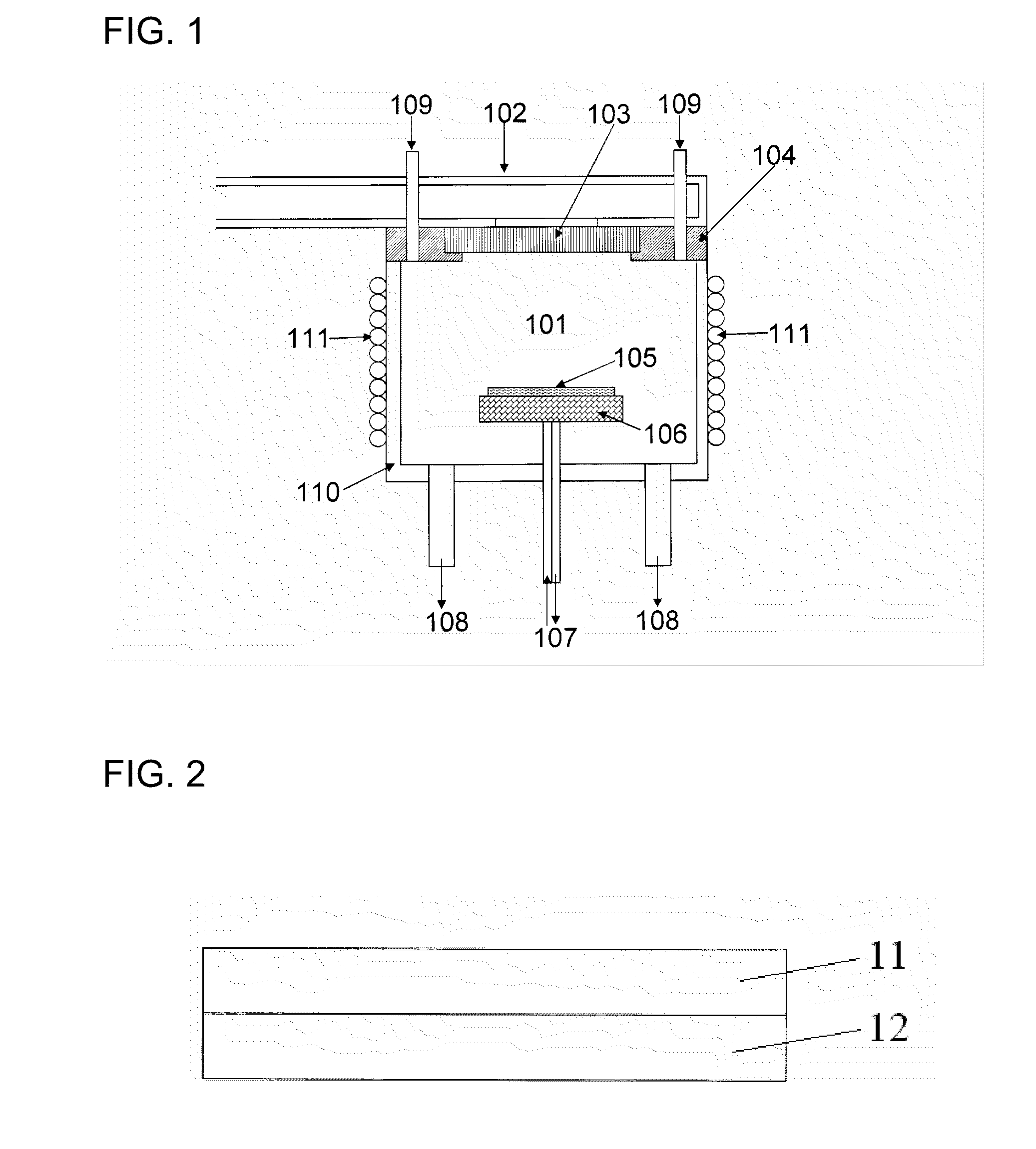
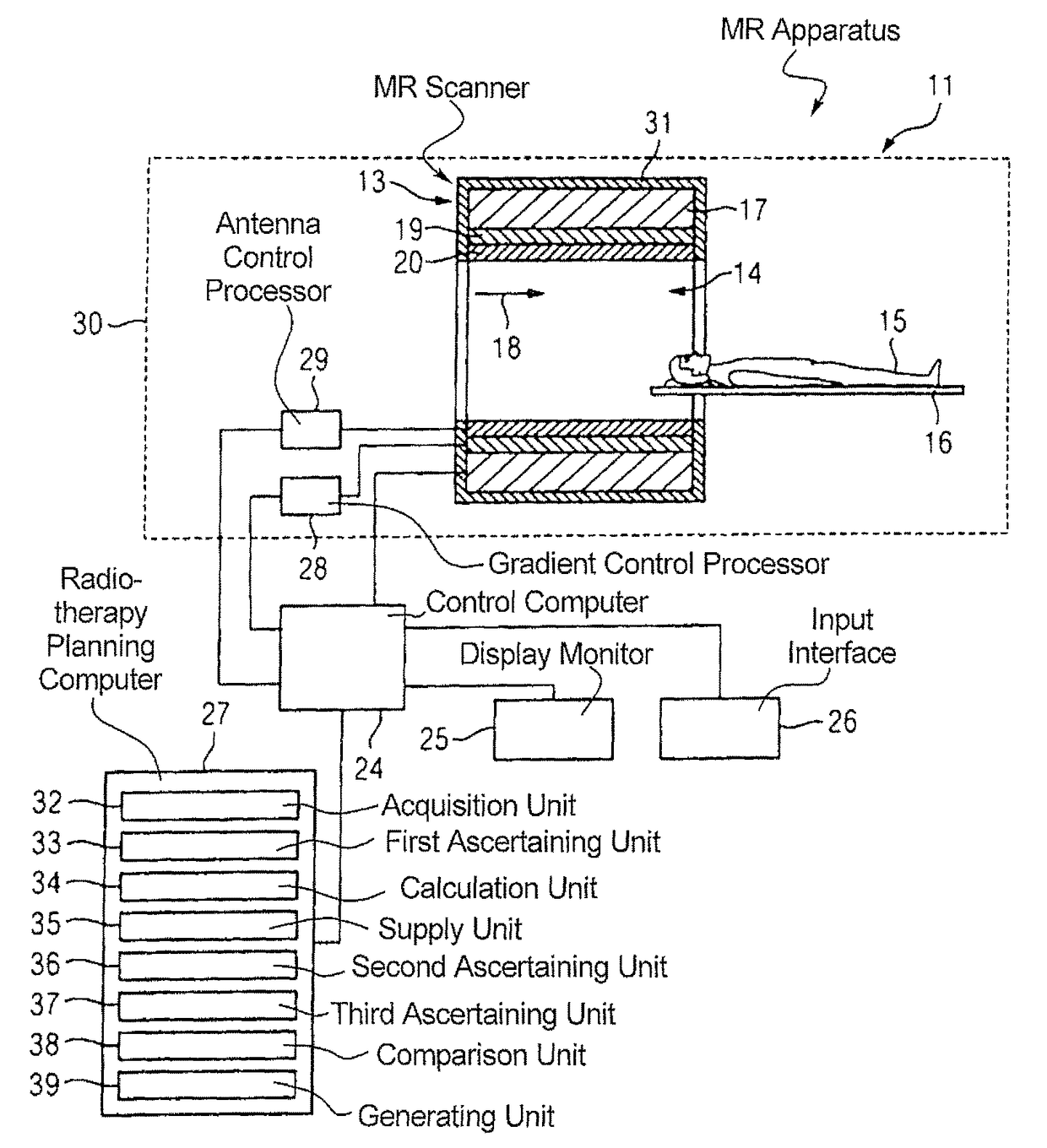
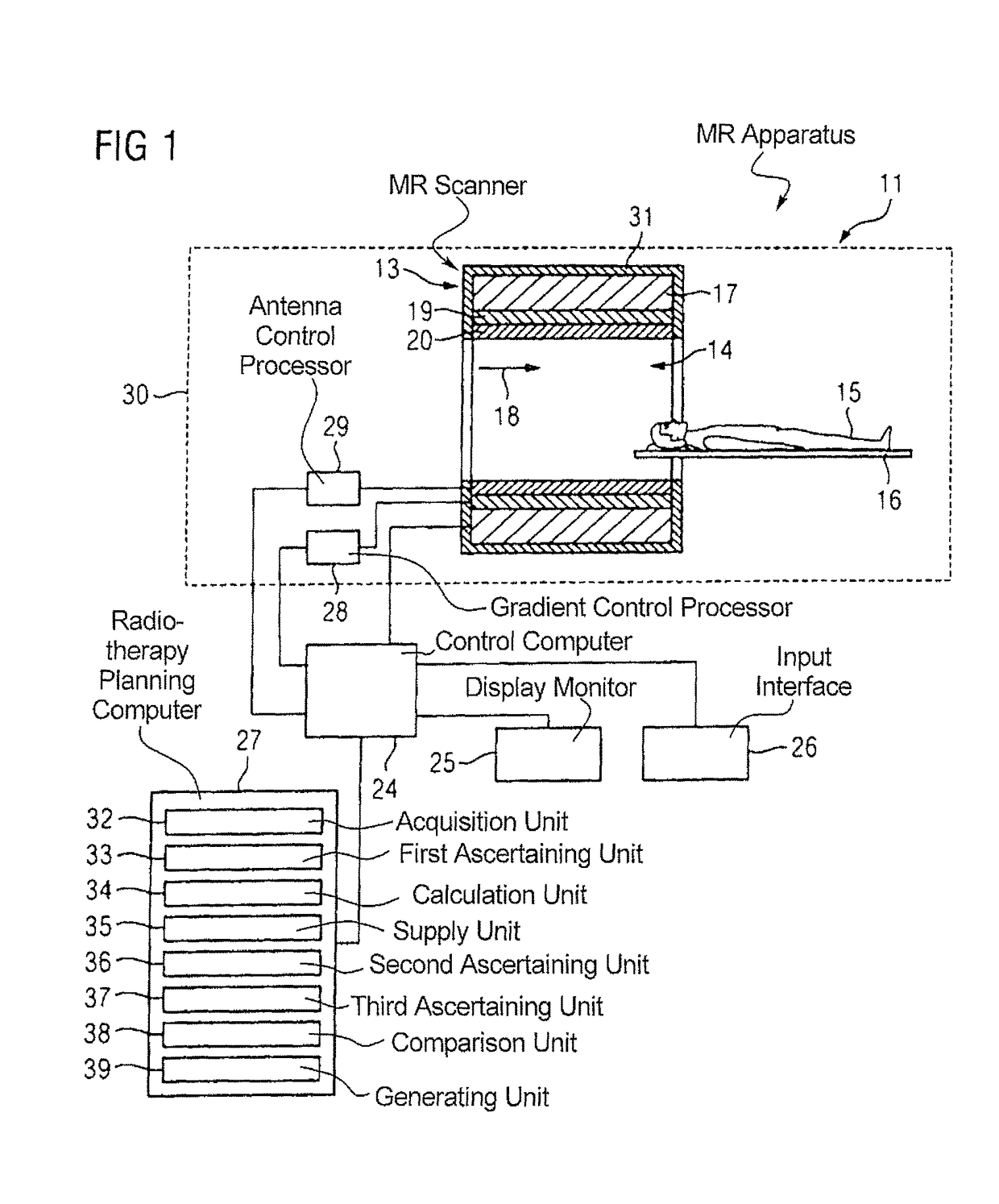
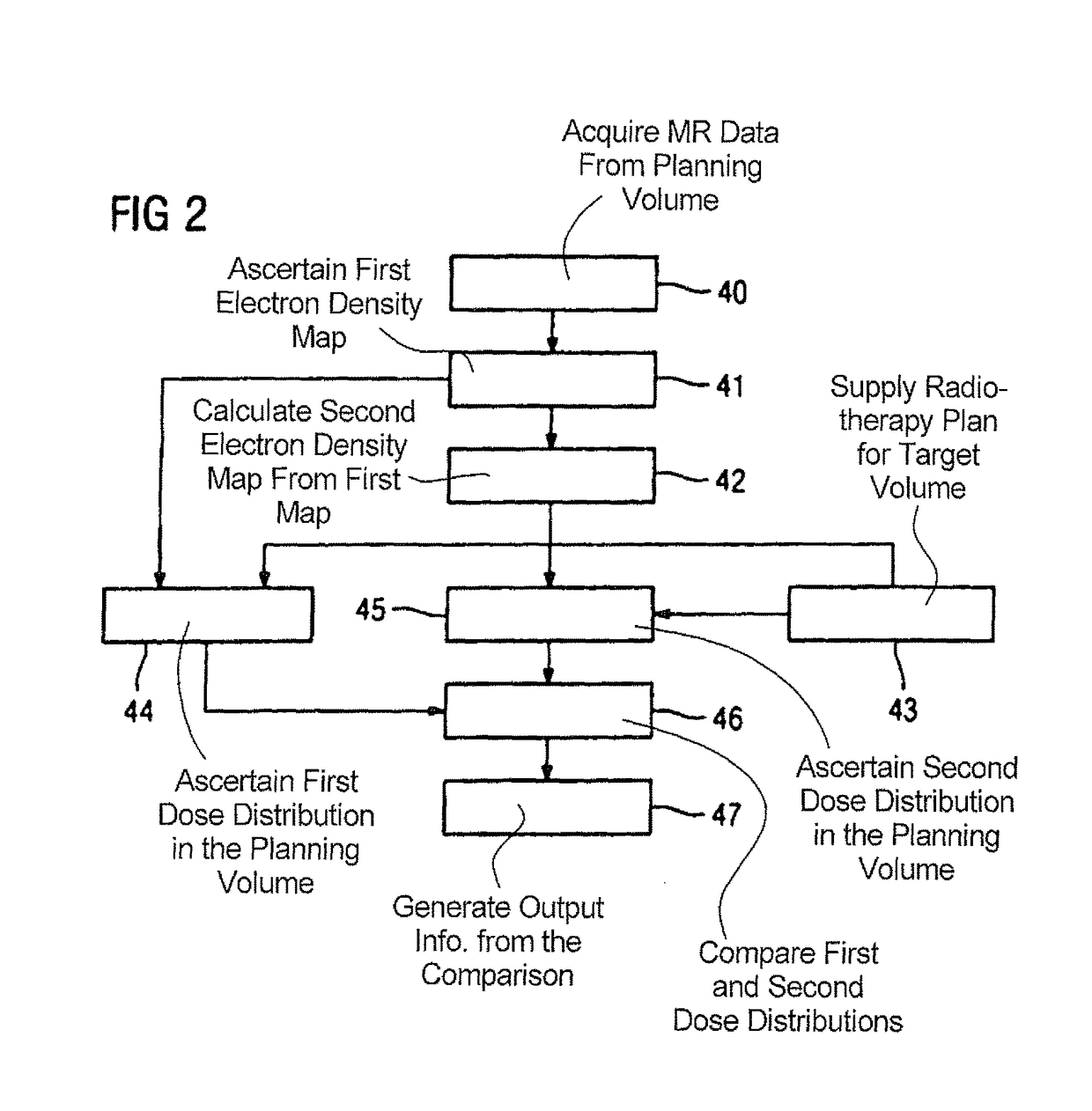
Method and magnetic resonance apparatus for quality control in planning radiotherapy of a patient
In a quality control method and computer for planning radiotherapy of patient, magnetic resonance (MR) image data, acquired from a planning volume of a patient, are provided to a computer and are used in the computer to generate a first electron density map of the planning volume. A second electron density map is generated using the first electron density map, wherein a value of electron density for a bone region in the planning volume is reduced compared to the first electron density map. First and second radiation dose distributions in the planning volume are respectively determined from the radiotherapy plan and the first electron density map, and the radiotherapy plan and the second electron density map. These distributions are compared in order to generate output information.
Owner:SIEMENS HEALTHCARE GMBH
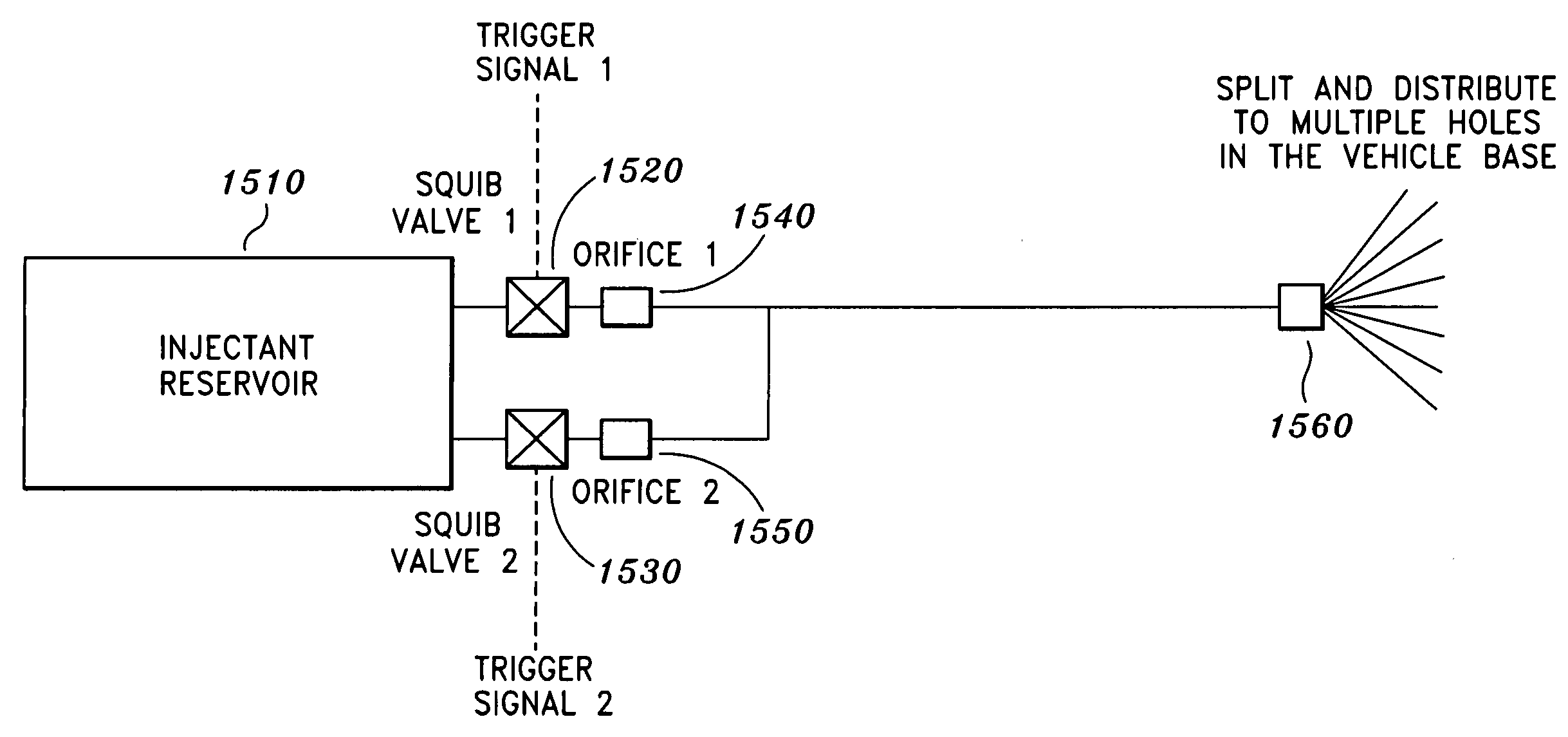
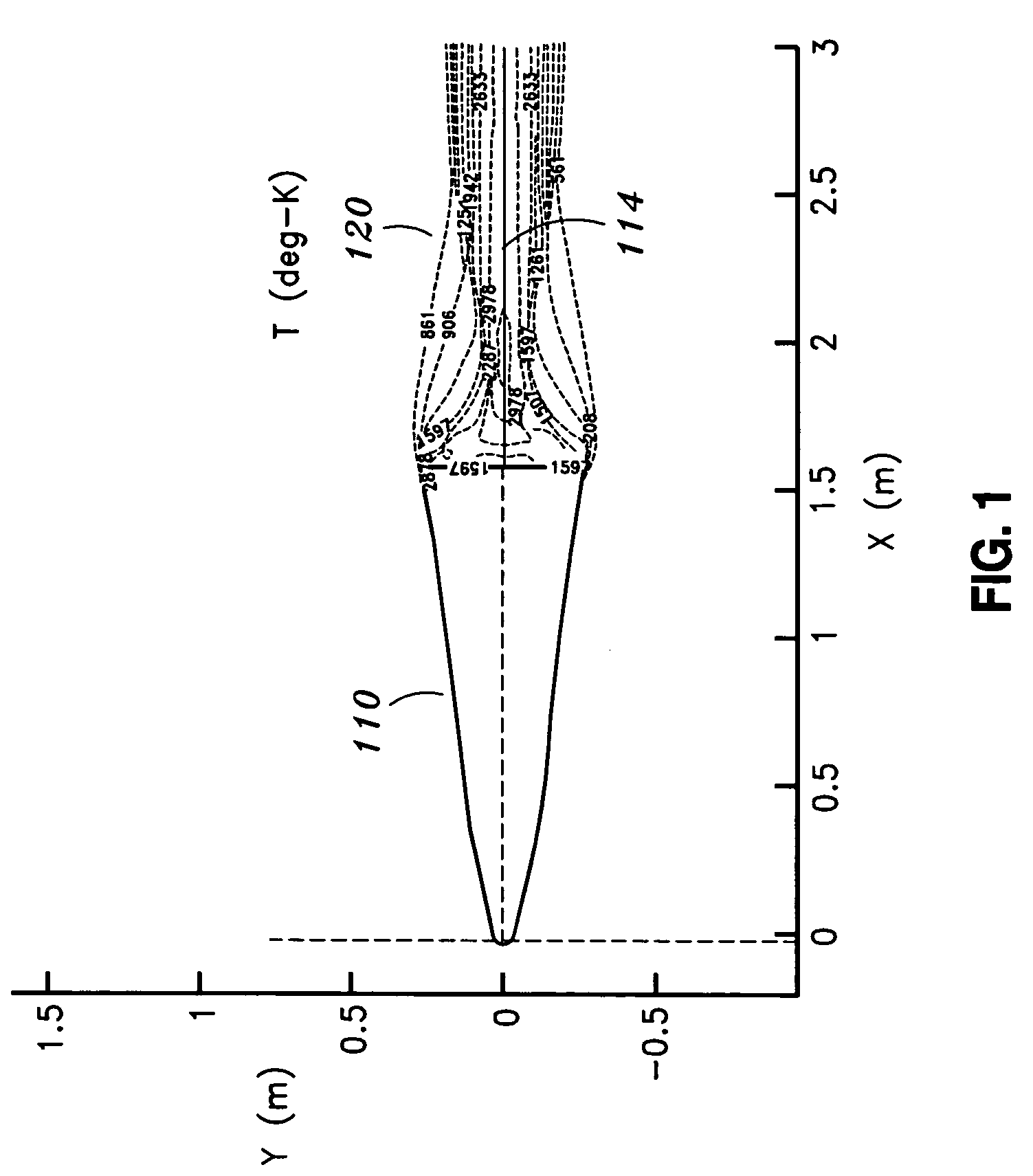
Method and system for providing cruciform steered, bent biconic and plasma suppression for maximum accuracy
InactiveUS7267303B1Reducing plasma induced communication disruptionReduction of free electronCosmonautic vehiclesSystems for re-entry to earthCruciformNose
A system for reducing plasma induced communication disruption is disclosed. The system utilizes an electrophilic gas injectant, bent reentry vehicle nose shaping and a number of flaps configured to provide 3-axis steering capability. Injecting electrophilic gas injectant into plasma accomplishes reduction of plasma induced communication disruption by removing free electrons in the plasma which causes the communication disruption. For reentry vehicles, a bent biconic nose also reduces plasma effect during reentry. By combining electrophilic gas injection and bent nose shaping, the system reduces and / or eliminates plasma induced communication disruption, such as, GPS communication blackout, for a reentry vehicle during reentry. Furthermore, the 3-axis steering capability renders the reentry vehicle more maneuverable. Performance of the reentry vehicle during reentry, such as, target accuracy, is thus improved.
Owner:LOCKHEED MARTIN CORP
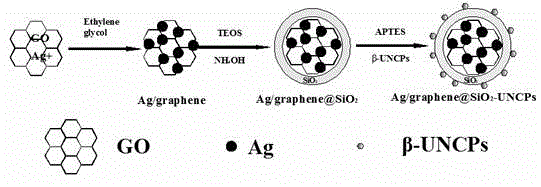
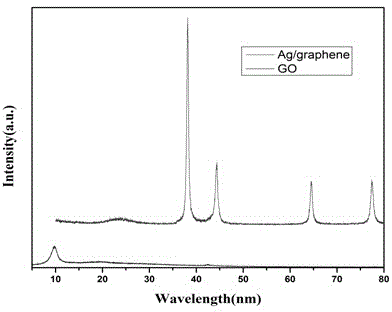
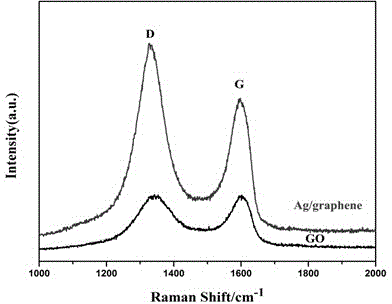
Silver/graphene-coated silicon dioxide composite upconversion nanocrystal and preparation method thereof
ActiveCN104946236AGood fluorescent effectHigh luminous intensityMaterial nanotechnologyNanoopticsField effectElectron density
The invention relates to a silver / graphene-coated silicon dioxide composite upconversion nanocrystal and a preparation method thereof. The nanocrystal structure of the obtained silver / graphene@SiO2-NaLuF4:Yb,Gd,Er composite fluorescent nanoparticles is as follows: silver-on-graphene is used as an inner core and coated with silicon dioxide, and the upconversion nanocrystal is grafted onto the silicon dioxide surface. The metal reinforced fluorescence effect of the noble metal silver is utilized to enhance the luminescent intensity of the upconversion nanocrystal. The silver is supported on the graphene to well prevent the silver particles from aggregation, and the silver nanoparticles, which are dispersed and fixed to the graphene, generate field effect superposition due to short distance, so that the mutual polarity and surface plasma resonance are mutually reinforced. Since the graphene is an excellent electron acceptor and conductor, after the charges of the silver nanoparticles transfer to the graphene, the electron density becomes lower, the effective dielectric constant increases, and thus, the surface plasma resonance of the nano silver is enhanced, so that the metal reinforced fluorescence effect of the nano silver becomes higher. The method enhances the upconversion luminescent intensity of the rare-earth upconversion nanocrystal by about 50 times.
Owner:SHANGHAI UNIV
Functional materials with reversible crosslinking
InactiveUS8916635B2Quick activationReduce electron densityAdhesive processesOrganic non-macromolecular adhesiveAdhesiveRoom temperature
The present invention relates to an innovative method for the reversible crosslinking of, for example, adhesives or coating materials. The reversible crosslinking method allows very rapid crosslinking even at room temperature and undoing of the crosslinks at higher temperatures, thereby regaining the capacity for thermoplastic processing and allowing the originally bonded substrates to be separated from one another again with ease. A particular aspect in this context is that a plurality of cycles of crosslinking and undoing of the crosslinks are possible with the present system.
Owner:EVONIK OPERATIONS GMBH
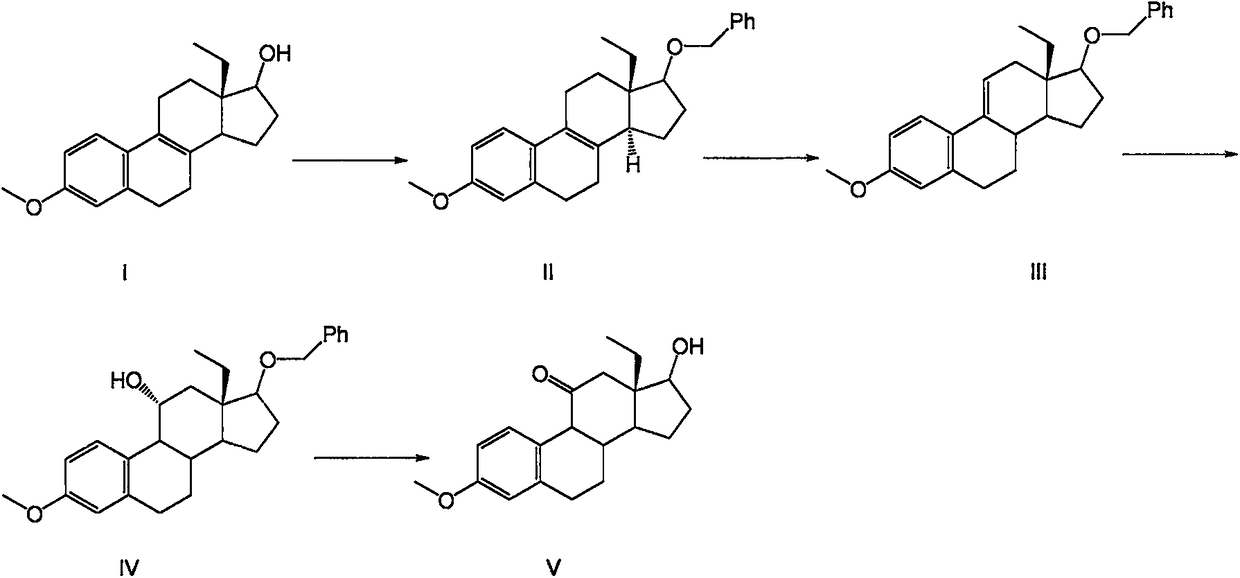
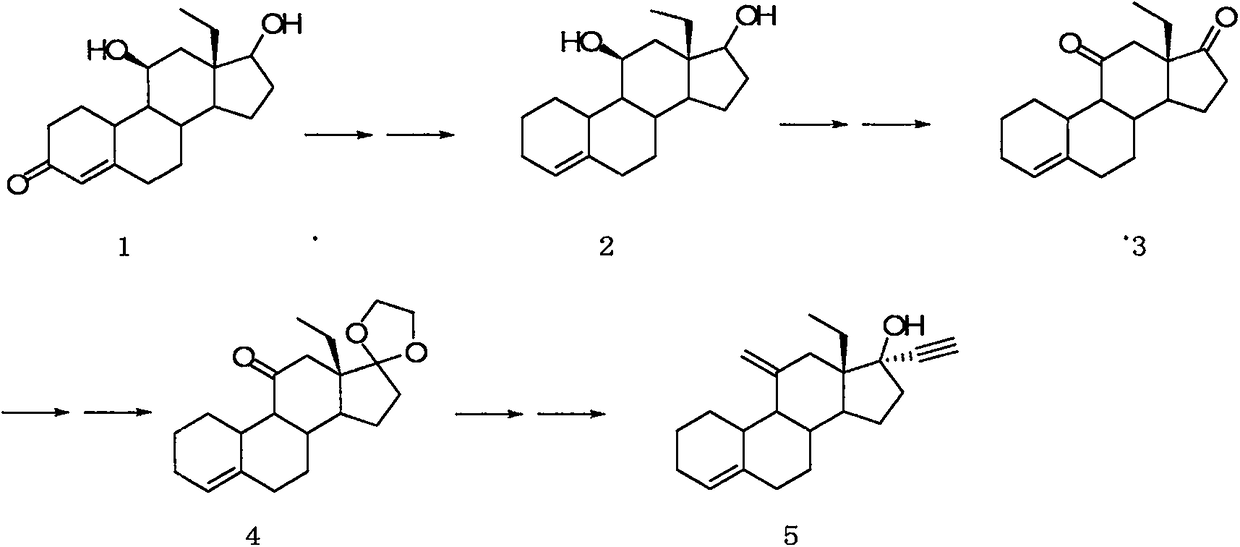
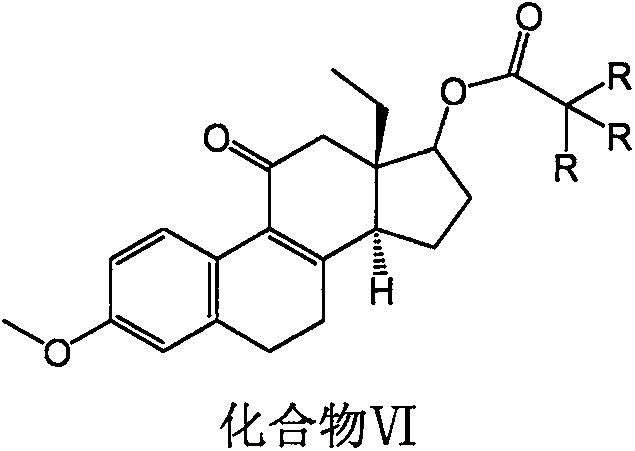
Desogestrel intermediate and preparation method thereof
InactiveCN109384824AThe chemical synthesis process is simpleEasy to operateSteroidsHydrolysisReagent
The invention relates to a steroid compound, i.e., a desogestrel intermediate (a compound VI), and a preparation method thereof. According to the method, 13beta-ethyl-3-methoxy-estra-1,3,5(10),8(9)-tetraen-17beta-ol is uses as a starting material and subjected to protection, epoxidation, hydrolysis, epoxidation and hydrolysis to produce the target product. The route of the method is simple and easy; chemical reagents used in the method are relatively economical and environmentally-friendly; and the method is simple in process, high in yield and suitable for industrial production. In the formula VI which is described in the specification, R is A and -CH3, or B and -Cl.
Owner:CHINA RESOURCES ZIZHU PHARMA
High-voltage lithium ion battery
PendingCN112186190AGuaranteed oxidation resistanceInterface Modification ConsolidationCell electrodesSecondary cellsElectrolytic agentLithium-ion battery
The invention discloses a high-voltage lithium ion battery, which comprises a positive electrode, a negative electrode and an electrolyte, wherein the positive electrode is a high-voltage fluorinatedpositive electrode, the negative electrode is a carbonaceous negative electrode, and the electrolyte uses fluorinated ether as a cosolvent and uses fluorinated carbonate and a boron-containing substance as additives. According to the battery disclosed in the invention, the oxidation resistance of the positive electrode material and the electrolyte can be improved, and the high-voltage stability ofan interface constructed by the positive electrode, the negative electrode and the electrolyte can be ensured, so that the key problem of the lithium ion battery under a high-voltage working condition is effectively solved, and the high-voltage cycle performance of the lithium ion battery is integrally improved.
Owner:XIAN UNIV OF SCI & TECH
Blue-light-emitting iridium complex, iridium complex monomer, phosphorus polymer, and organic electroluminescence device using same
ActiveUS20120138917A1Increase blue-light-emitting efficiencyImprove stabilityIndium organic compoundsSolid-state devicesIridiumPhotopolymer
Provided are a blue-light-emitting iridium complex, an iridium complex monomer, a phosphorescent polymer, and an organic electroluminescent device using same. The blue-light-emitting iridium complex contains a ligand having a low electron density structure, such as triazole or tetrazole. The iridium complex monomer containing a ligand having a polymerizable vinyl group produces a blue phosphorescent polymer through the polymerization with carbazole derivatives. The organic electroluminescent device comprises a first electrode, a second electrode, and a light-emitting layer interposed between the first electrode and the second electrode, wherein the light-emitting layer contains the above-described iridium complex or polymer containing the iridium complex.
Owner:GWANGJU INST OF SCI & TECH
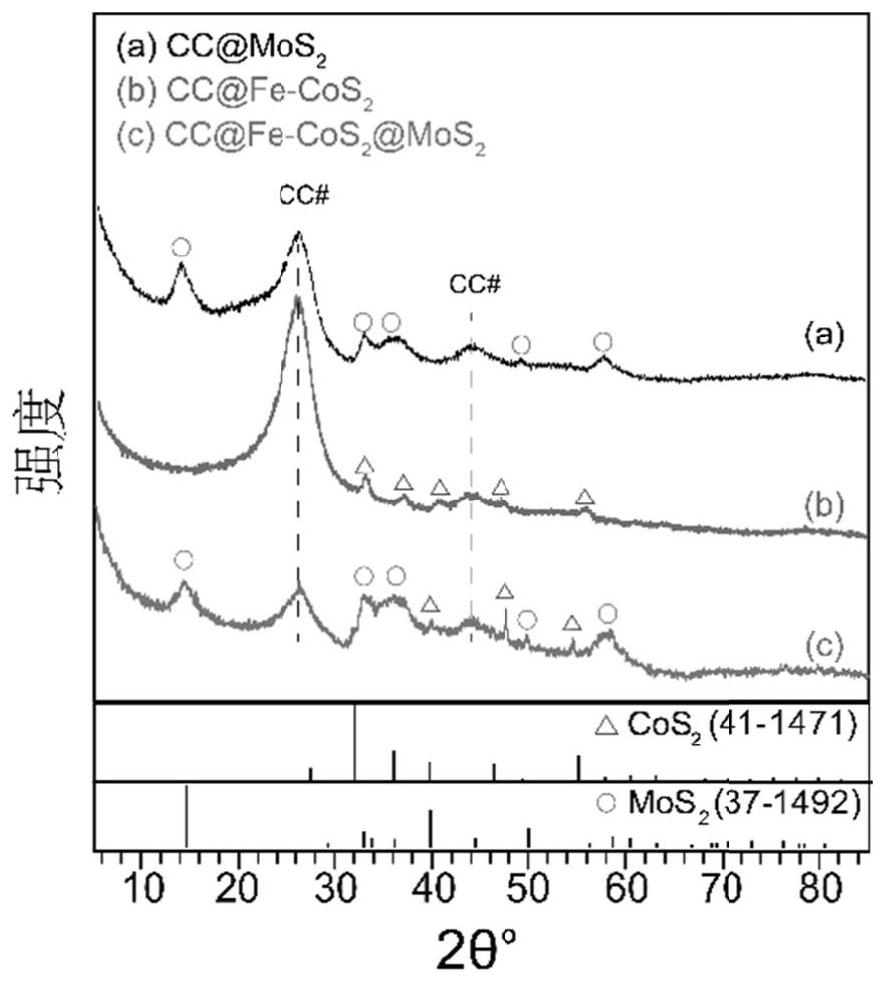
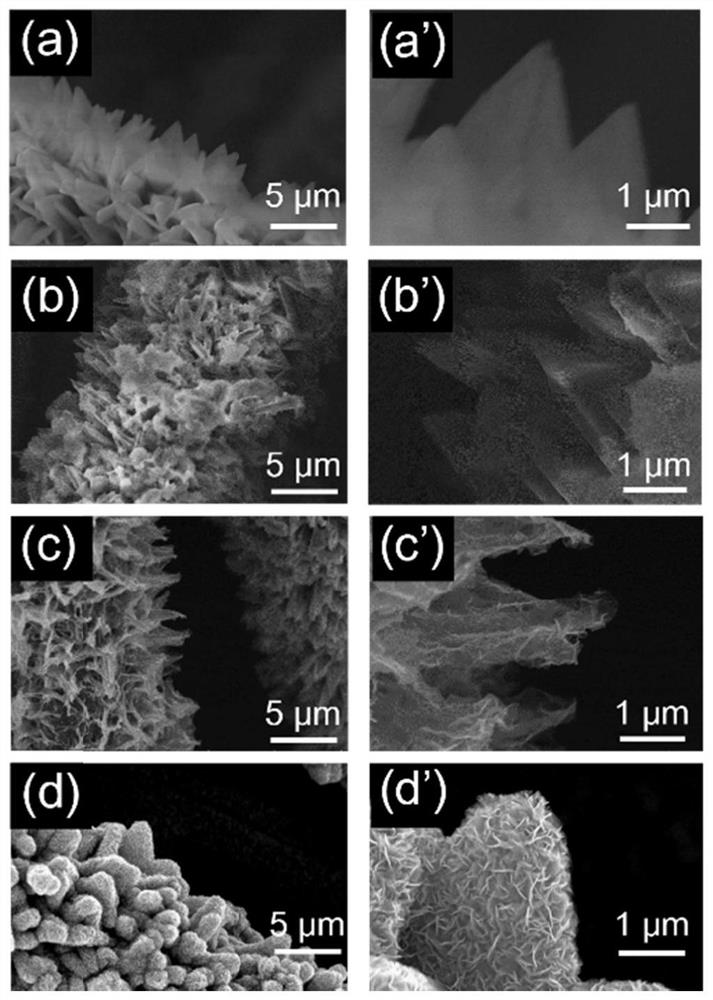
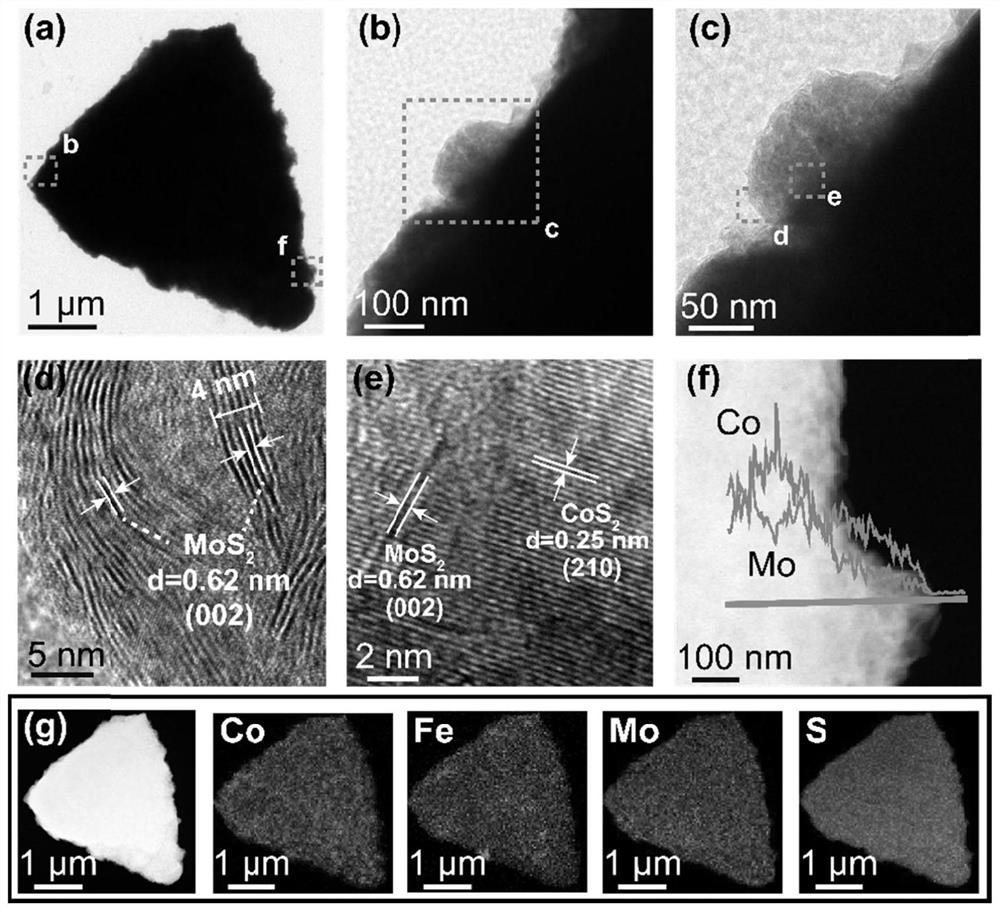
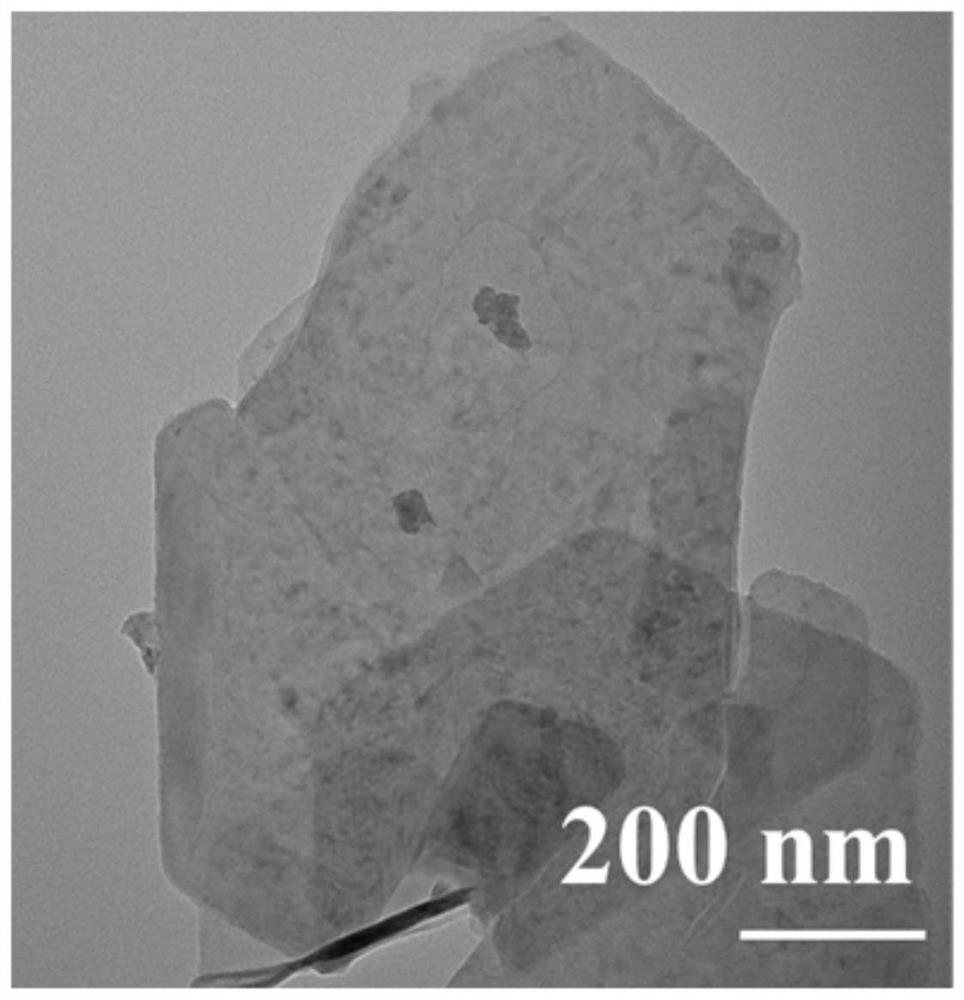
Triangular nano array assembled by iron-doped cobalt sulfide and molybdenum sulfide nanosheets as well as preparation method and application of triangular nano array
ActiveCN113235128AImprove electrochemical stabilityImprove conductivityMaterial nanotechnologyElectrodesSulfidationElectrochemistry
The embodiments of the invention provides a triangular nano array assembled by iron-doped cobalt sulfide and molybdenum sulfide nanosheets. According to the triangular nano array assembled by the iron-doped cobalt sulfide and molybdenum sulfide nanosheets, carbon cloth serves as a substrate, the iron-doped cobalt sulfide grows on the carbon cloth to form the triangular nano array, and the molybdenum sulfide nanosheets grow on the triangular nano array formed by the iron-doped cobalt sulfide. According to the triangular nano array assembled by the iron-doped cobalt sulfide and molybdenum sulfide nanosheets, the carbon cloth is used as an acid-resistant and alkali-resistant conductive substrate, so that the conductivity of the material is improved, and the chemical stability of the material is also improved; Fe is doped into CoS2 to obtain Fe-CoS2, the electronic structure of CoS2 is optimized through electronic regulation and control, and the HER catalytic activity of the material is improved; the MoS2 nanosheet is an ultrathin nanosheet, so that more catalytic active sites are exposed; and the triangular nano array assembled by the iron-doped cobalt sulfide and molybdenum sulfide nanosheets has excellent HER catalytic activity and lasting electrochemical stability.
Owner:BEIJING NORMAL UNIVERSITY +1
Charge exchange device
InactiveUS20100272977A1High strengthReduce intensityLayered productsHandling using charge exchange devicesCharge exchangeCarbon nanotube
The present invention provides a charge exchange member having a new function, which solves problems of fragility of a diamond thin film and a low electron density of a CNTS that are challenges of a charge exchange foil. The present invention relates to a charge exchange device comprising a diamond thin film and a non-woven carbon nanotube sheet, in which the diamond thin film is deposited on the non-woven carbon nanotube sheet.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method and magnetic resonance apparatus for quality control in planning radiotherapy of a patient
ActiveUS9878178B2Easily decideLow effortMeasurements using NMR imaging systemsX-ray/gamma-ray/particle-irradiation therapyProgram planningResonance
In a quality control method and computer for planning radiotherapy of patient, magnetic resonance (MR) image data, acquired from a planning volume of a patient, are provided to a computer and are used in the computer to generate a first electron density map of the planning volume. A second electron density map is generated using the first electron density map, wherein a value of electron density for a bone region in the planning volume is reduced compared to the first electron density map. First and second radiation dose distributions in the planning volume are respectively determined from the radiotherapy plan and the first electron density map, and the radiotherapy plan and the second electron density map. These distributions are compared in order to generate output information.
Owner:SIEMENS HEALTHCARE GMBH
Non-aqueous electrolyte secondary battery
ActiveUS7879489B2Large capacitySatisfactory characteristicOrganic electrolyte cellsSecondary cellsArameSolvent
To provide a high-capacity non-aqueous electrolyte secondary battery that exhibits satisfactory charge / discharge cycle characteristics even in a high temperature environment. The battery has: a positive electrode including a nickel-containing lithium composite oxide; a negative electrode capable of charging and discharging; a separator interposed between the positive and negative electrodes; and a non-aqueous electrolyte containing a non-aqueous solvent and a solute dissolved therein. The non-aqueous electrolyte contains a fluorine atom-containing aromatic compound. The nickel-containing lithium composite oxide is represented by, for example, LiNixM1-x-yLyO2 where element M is at least one selected from the group consisting of Co and Mn; element L is at least one selected from the group consisting of Al, Sr, Y, Zr, Ta, Mg, Ti, Zn, B, Ca, Cr, Si, Ga, Sn, P, V, Sb, Nb, Mo, W and Fe; and x and y satisfy 0.1≦x≦1 and 0≦y≦0.1.
Owner:PANASONIC CORP
Laminated packaging material
InactiveUS20090104468A1Good effectEffective protectionSynthetic resin layered productsThin material handlingLinear low-density polyethyleneInter layer
[Problems] To provide a laminated packaging material having a simplified lamination structure for a packaging material having the gas blocking capability, enhancing an inter-layer mechanical strength between a barrier layer and an adjoining layer, and capable of preserving the sealing strength of a sealant layer which is the innermost layer.[Solution] The laminated packaging material has an outer layer made from thermoplastic resin, a paper-made base layer, an EVOH gas barrier layer, and a sealant layer, in which the layer adjoining the gas barrier layer is made from a blend polymer in which 92 to 98 weight % of linear low density polyethylene and 2 to 8 weight % of thermoplastic elastomer are blended, and the thermoplastic elastomer has the affinity with a hydroxyl group and / or a polarized group in the EVOH layer.
Owner:TETRA LAVAL HLDG & FINANCE SA
Plasma filming apparatus, and plasma filming method
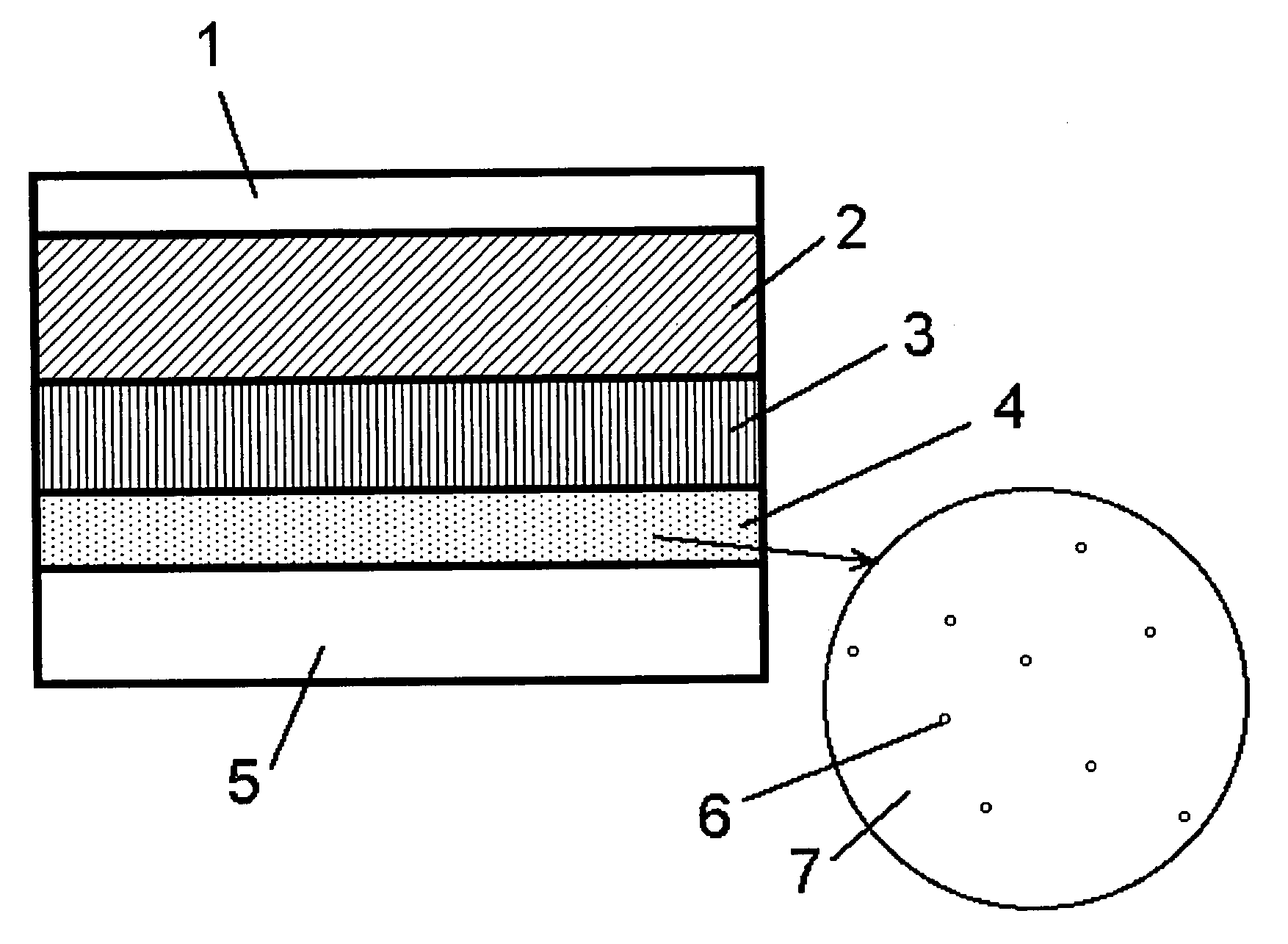
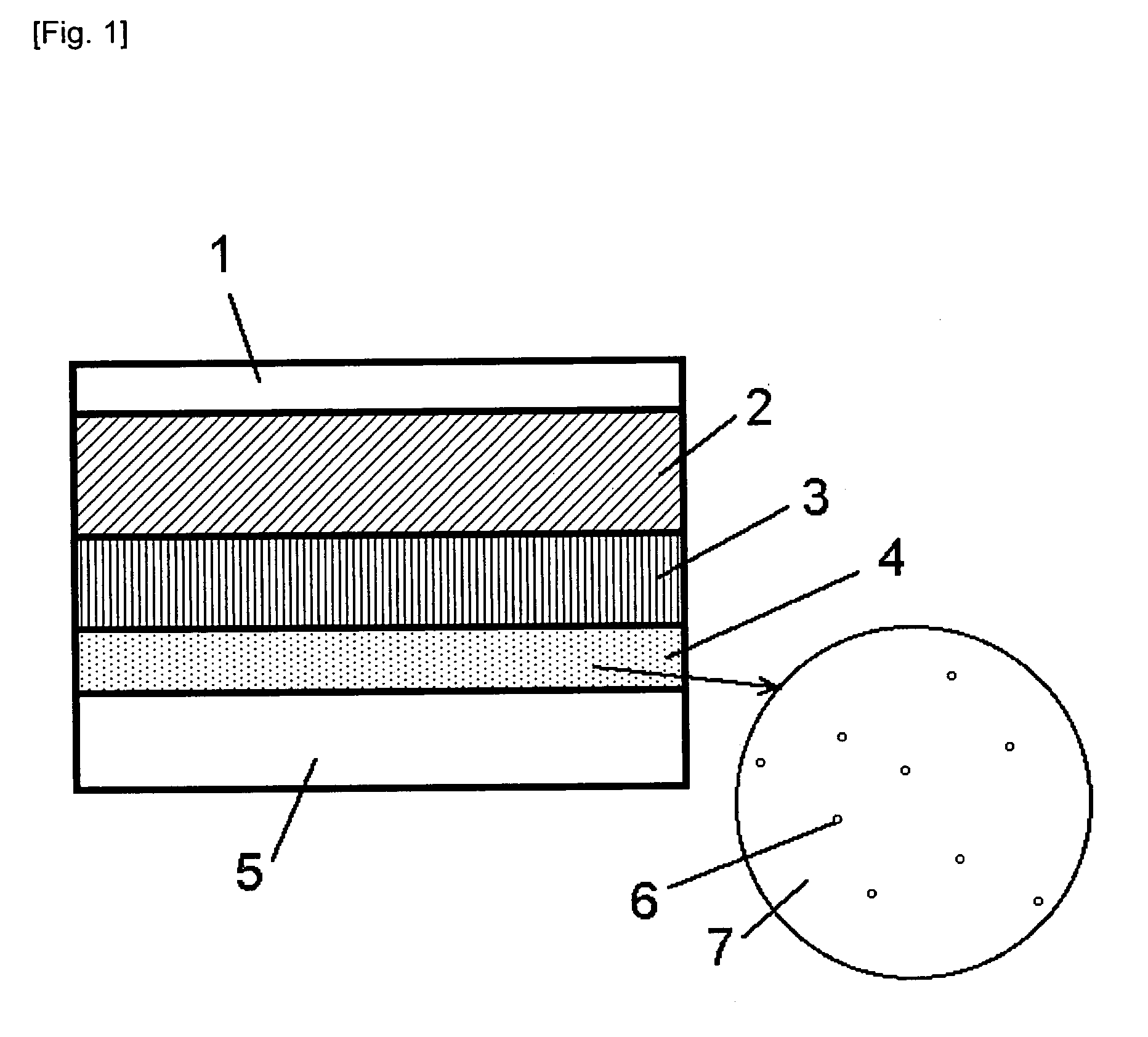
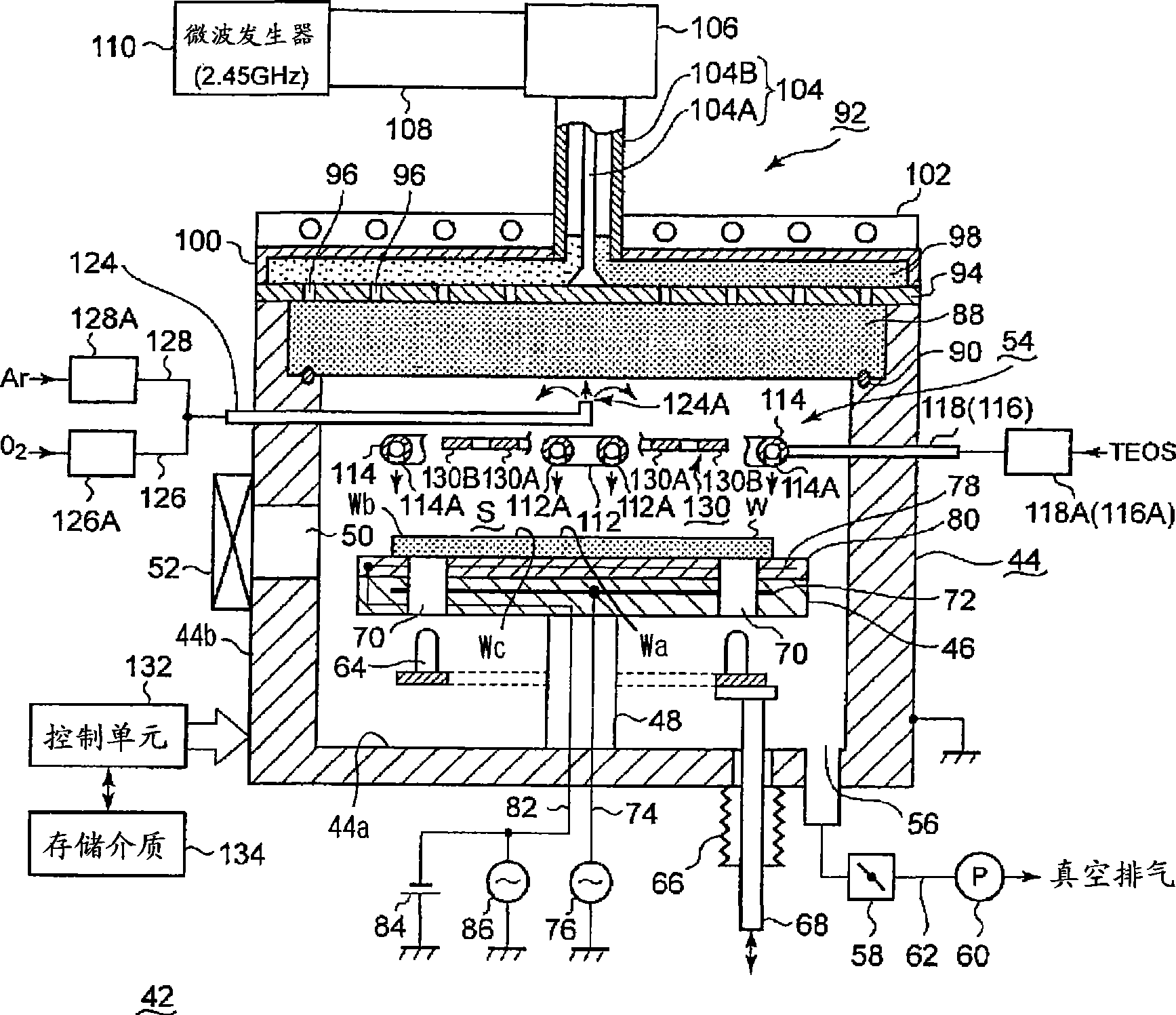
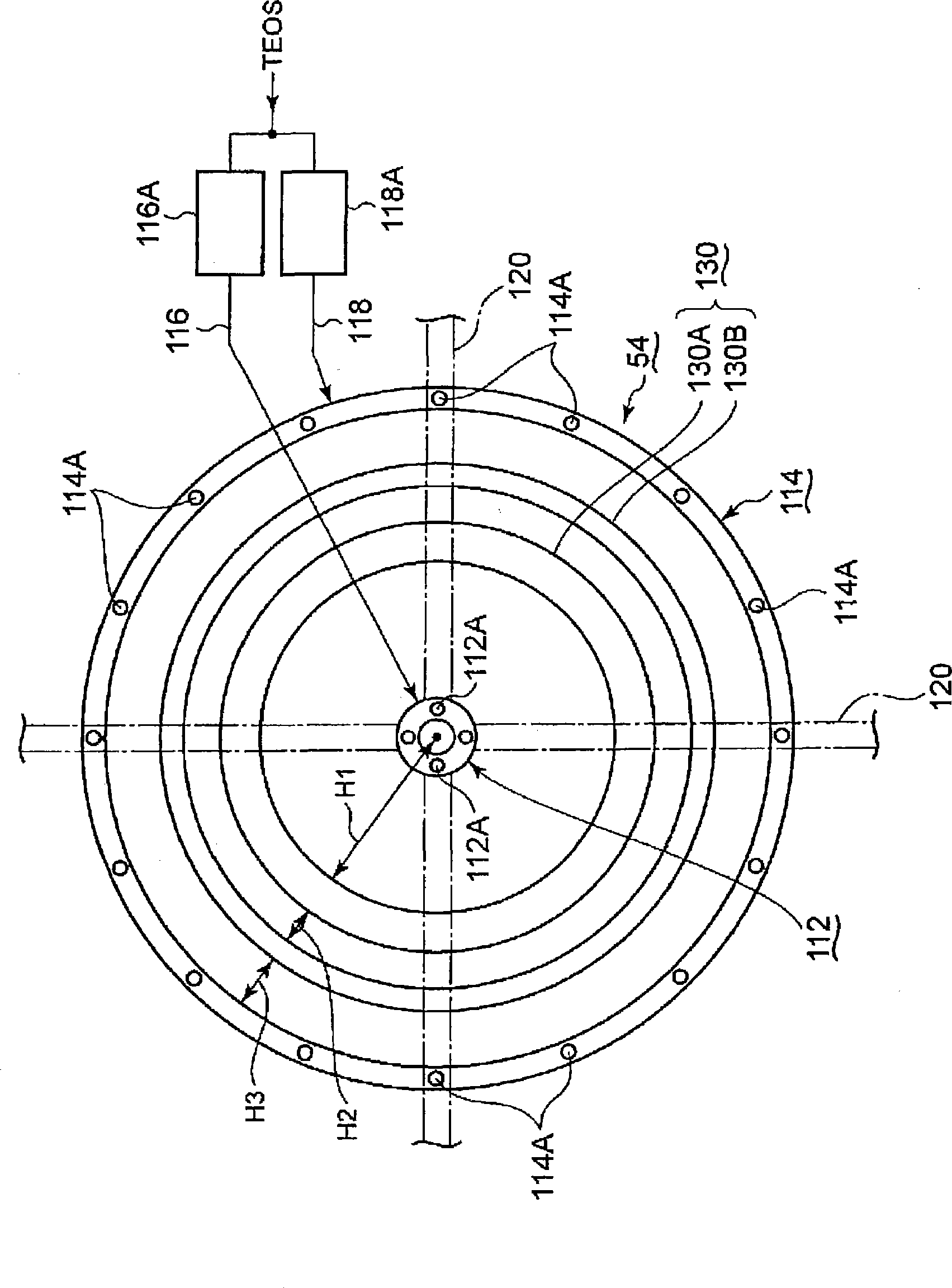
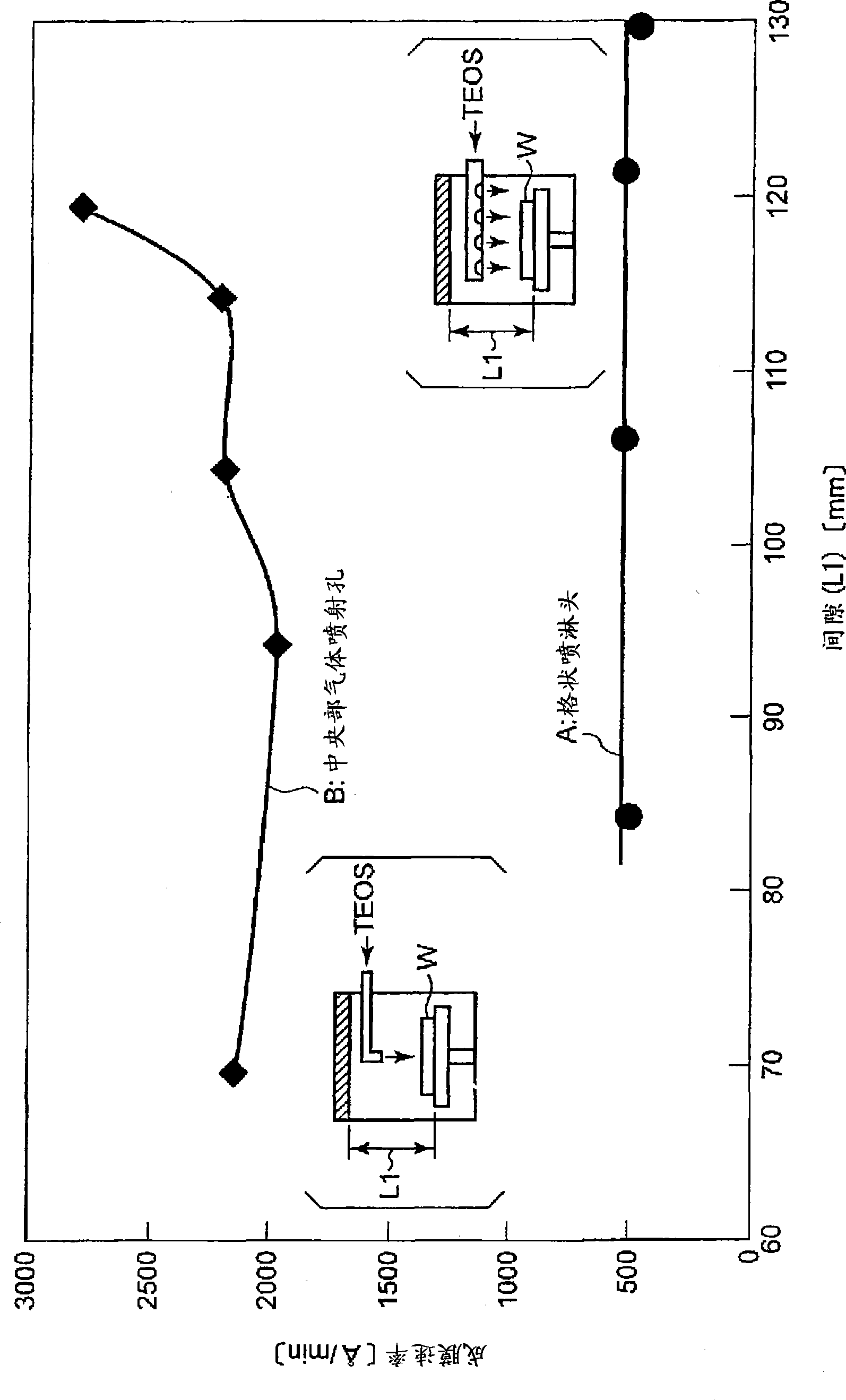
InactiveCN101523573AReduce electron densitySmall footprintElectric discharge tubesSemiconductor/solid-state device manufacturingIn planeMicrowave
Provided is a plasma filming apparatus, which can keep high not only a filming rate but also an in-plane homogeneity of a film thickness. The plasma filming apparatus comprises a treating container (44) made evacuative, a placing bed (46) for placing a treatment object (W) thereon, a ceiling plate (88) mounted in the ceiling and made of a dielectric material for transmitting microwaves, gas introducing means (54) for introducing a treating gas containing a filming raw gas and a support gas, and microwave introducing means (92) having a plain antenna member disposed on the ceiling side for introducing the microwaves. The introducing means includes central gas injection holes (112A) for the raw gas positioned above the central portion of the treatment object, and a plurality of peripheral gas injection holes (114A) for the raw gas arrayed above the peripheral portion of the treatment object and along the peripheral direction of the same. Above the treatment object and between the central gas injection holes (112A) and the peripheral gas injection holes (114A), there are disposed plasma shielding portions (130) for shielding the plasma along the peripheral direction.
Owner:TOKYO ELECTRON LTD
Iridium complex containing carbazole-substituted pyridine and phenyl derivatives as main ligand and organic light-emitting diodes containing the same
ActiveUS8232549B2Reduce electron densityIncrease electron densityElectroluminescent light sourcesSolid-state devicesIridiumDopant
The present invention relates to a novel iridium complex into which carbazole-substituted pyridine derivatives and various substituents-substituted phenyl derivatives are introduced as main ligand and a electrophosphorescence diode containing the same as a dopant of a light-emitting layer. When the iridium complex according to the present invention is applied to an organic light-emitting diode, the heat-resistance property and the light-emitting property can be significantly improved as well as the light-emitting efficiency and the like can be significantly improved by doping the iridium complex compound into the light-emitting layer as compared to the conventional organic light-emitting diode.
Owner:INKTEC CO LTD +1
Organic electroluminescence device
ActiveUS8187729B2Increase productionIncreased durabilityDischarge tube luminescnet screensElectroluminescent light sourcesPlatinum complexPlatinum salts
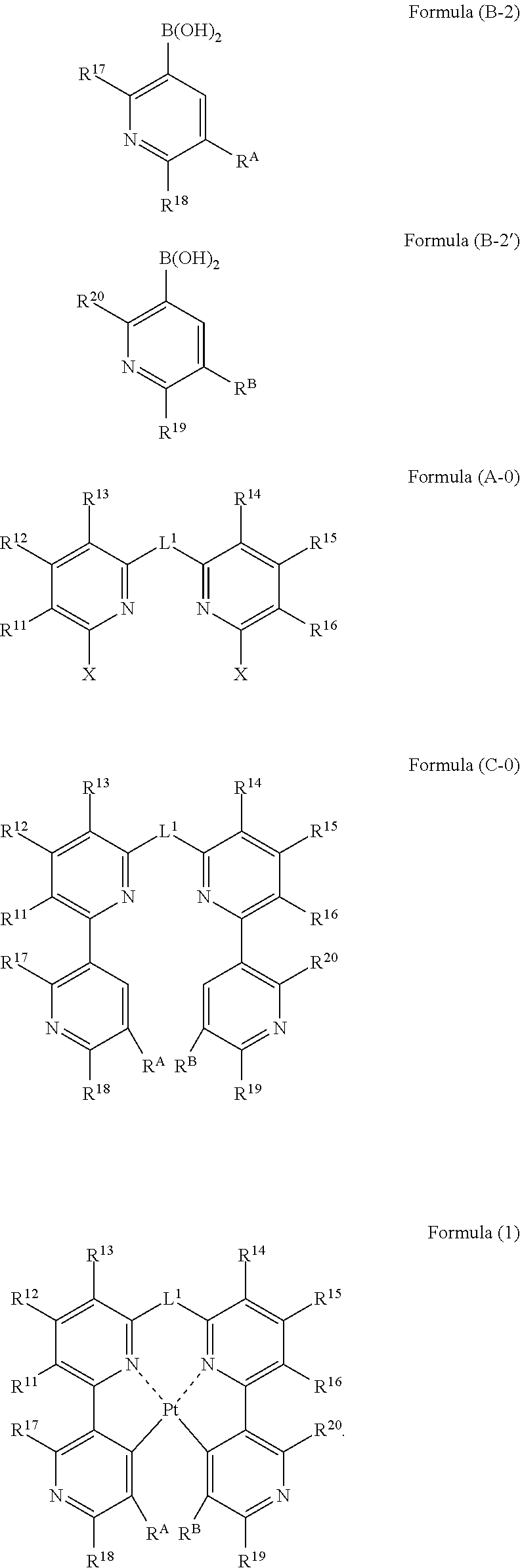
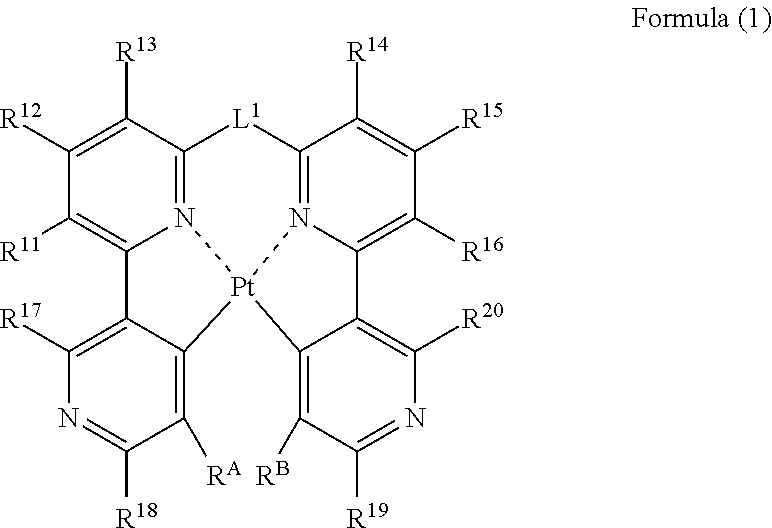
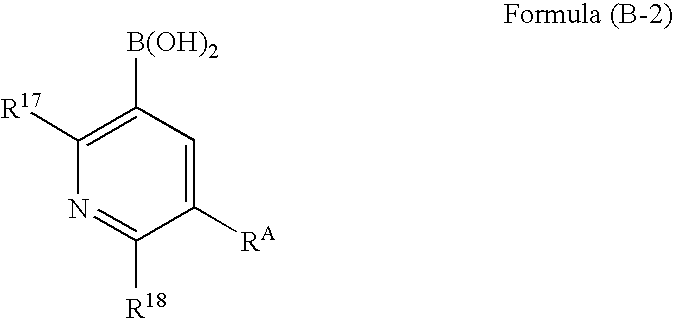
A process for preparing a platinum complex represented by the following formula (1) includes reacting a compound represented by the following formula (B-2) and a compound represented by the following formula (B-2′) with a compound represented by the following formula (A-0) to obtain a compound represented by the following formula (C-0); and reacting the compound represented by the formula (C-0) with a platinum salt:
Owner:UDC IRELAND
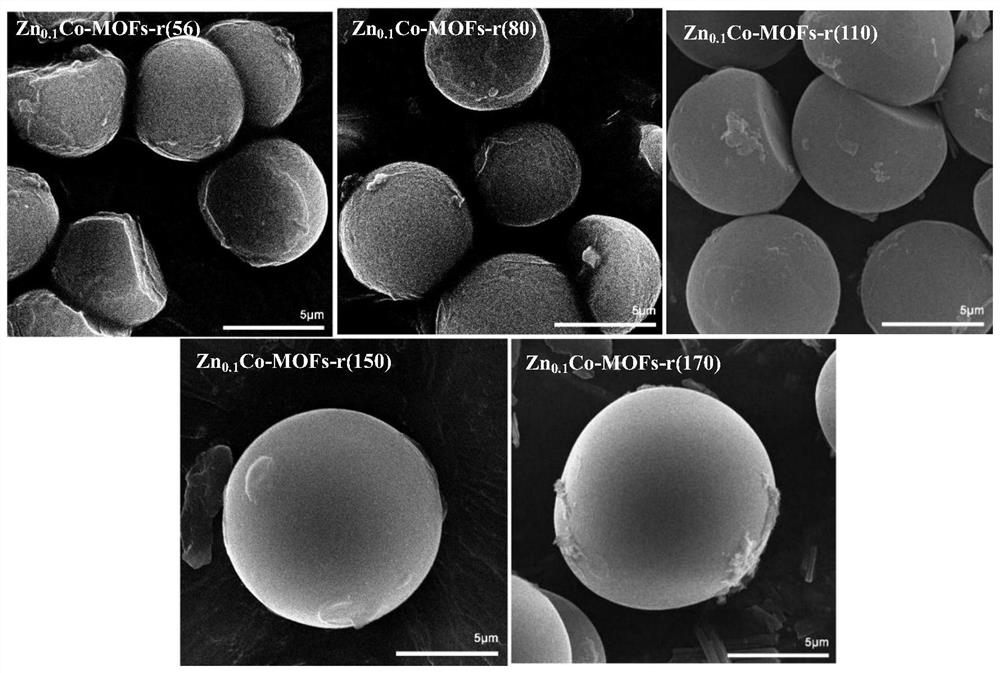
Method for preparing spherical bimetallic MCo-MOFs catalytic material through microwave-ultrasonic wave synergistic assistance
ActiveCN112452357AFast and even heatingEnhanced mass transferOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCobalt saltDicarboxylic acid
The invention discloses a method for preparing a spherical bimetallic MCo-MOFs catalytic material through microwave-ultrasonic synergistic assistance, which comprises the following steps: firstly, preparing a spherical bimetallic MCo-MOFs precursor through synergistic assistance of a metal salt I (metal cobalt salt), a metal salt II and aromatic dicarboxylic acid in a microwave-ultrasonic combinedmode; and further the precursor solution is crystallized through a hydrothermal rotation method to prepare the spherical bimetallic MCo-MOFs catalytic material. The method is simple in process, low in cost, convenient to operate and high in yield, and the prepared product is uniform in particle size, controllable in form and high in catalytic activity.
Owner:HUBEI UNIV
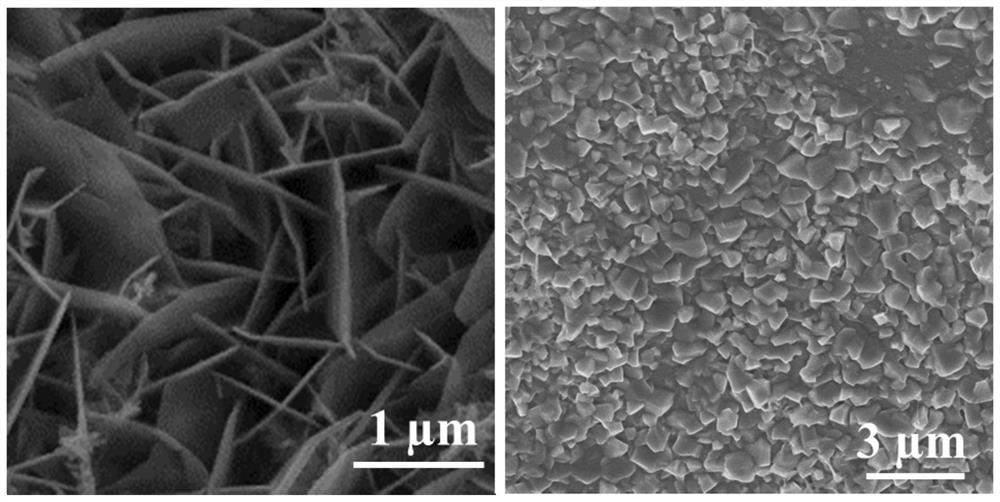
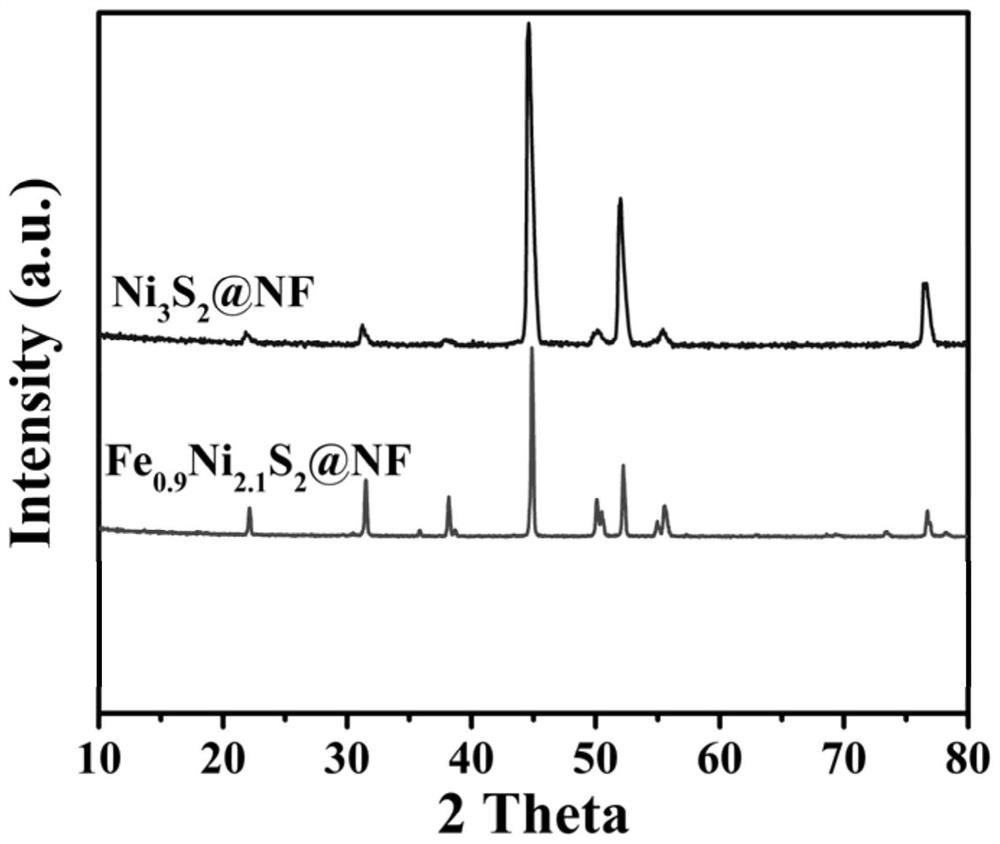
Ternary metal sulfide nanosheet material and preparation and water electrolysis application thereof
ActiveCN111774071AReduce the free energy of adsorptionLarge specific surface areaMaterial nanotechnologyCatalyst activation/preparationPtru catalystMetallic sulfide
The invention relates to a ternary metal sulfide nanosheet material and preparation and water electrolysis application thereof. The preparation method of the material comprises the following steps: 1)pretreating foam metal to obtain a matrix; 2) adding ferric chloride and sodium sulfide into water to obtain a mixed solution; and 3) putting the matrix into the mixed solution, carrying out hydrothermal reaction, washing and drying to obtain the ternary metal sulfide nanosheet material, the material is used as a catalyst for water electrolysis reaction. Compared with the prior art, the ternary metal sulfide nanosheet material prepared by the method is excellent in electro-catalytic performance, simple in preparation process and low in cost, can be used for carrying out stable and efficient hydrogen evolution reaction, oxygen evolution reaction and full-water decomposition under different current densities, and has a huge potential value in large-scale hydrogen production application.
Owner:FUDAN UNIV
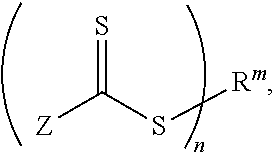
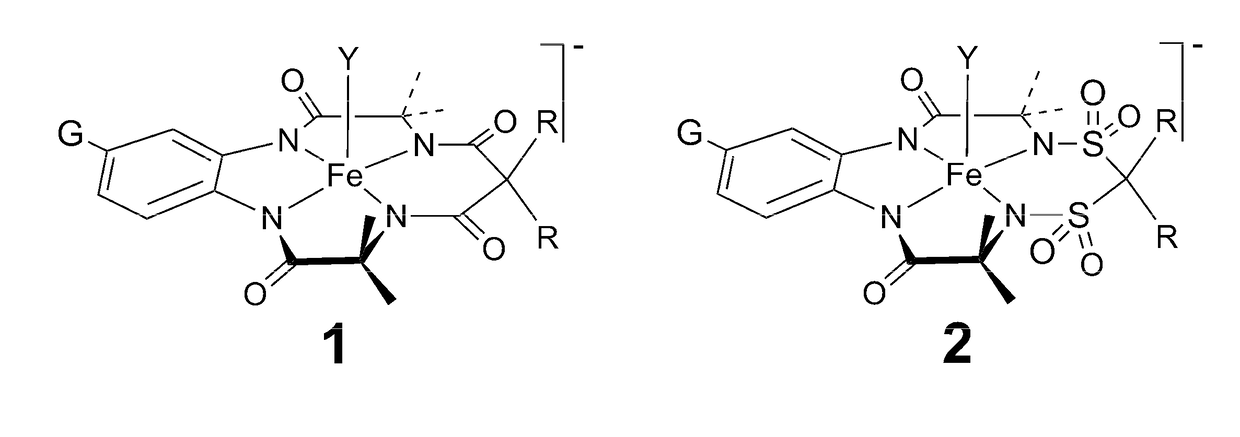
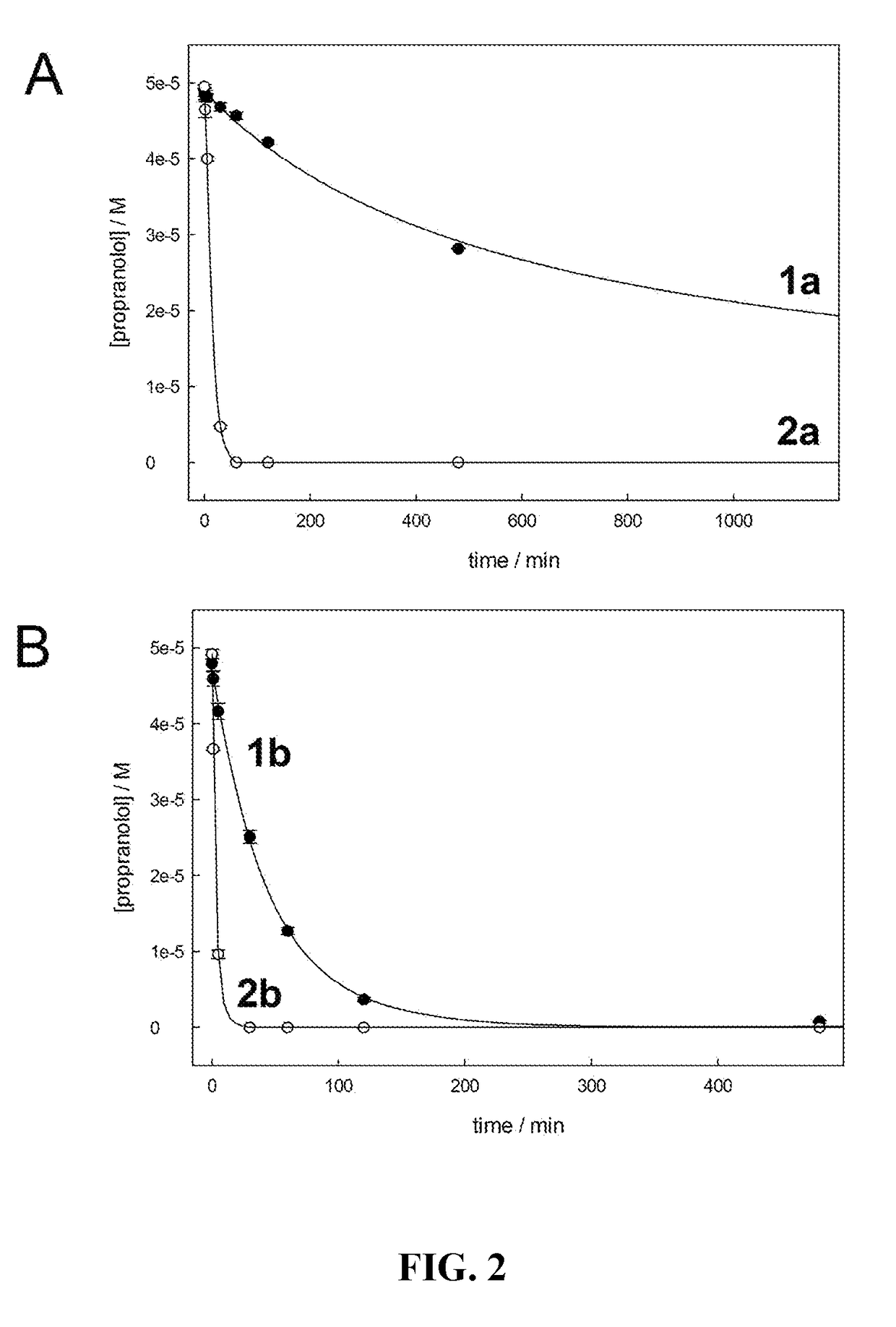
Far superior oxidation catalysts based on macrocyclic compounds
ActiveUS20180304247A1Easy accessEfficient oxidative catalysisGroup 1/11 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsAlkaline earth metalRare earth
An especially robust compound and its derivative metal complexes that are approximately one hundred-fold superior in catalytic performance to the previously invented TAML analogs is provided having the formula:whereinY1, Y2, Y3 and Y4 are oxidation resistant groups which are the same or different and which form 5- or 6-membered rings with a metal, M, when bound to D; at least one Y incorporates a group that is significantly more stable towards nucleophilic attack than the organic amides of TAML activators; D is a metal complexing donor atom, preferably N; each X is a position for addition of a labile Lewis acidic substituent such as (i) H, deuterium, (ii) Li, Na, K, alkali metals, (iii) alkaline earth metals, transition metals, rare earth metals, which may be bound to one or more than one D, (iv) or is unoccupied with the resulting negative charge being balanced by a nonbonded countercation; at least one Y may contain a site that is labile to acid dissociation, providing a mechanism for shortening complex lifetime. The new complexes deliver catalytic performances that promise to revolutionize multiple oxidation technology spaces including water purification.
Owner:CARNEGIE MELLON UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com