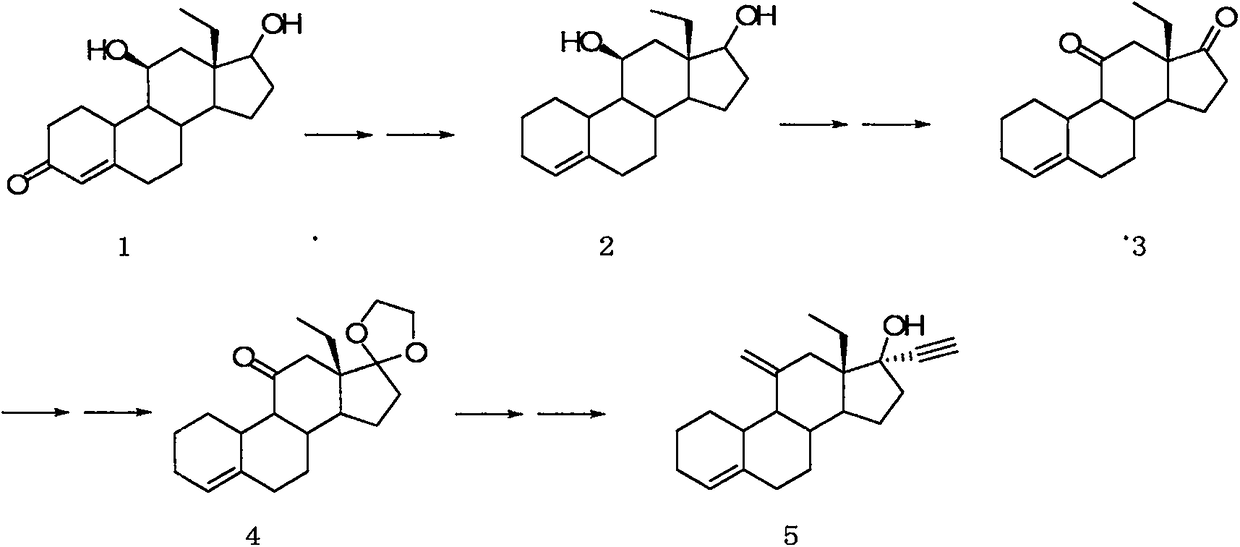
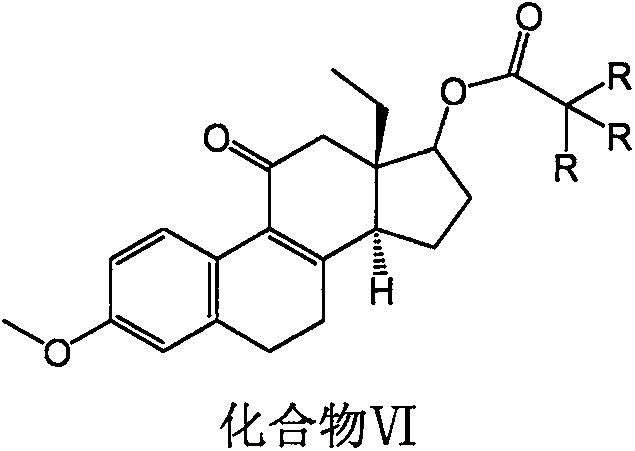
Desogestrel intermediate and preparation method thereof
A technology for desogestrel and intermediates, which is applied in the field of desogestrel intermediate compounds and their preparation, and can solve the problems of poor selectivity of the 17-position carbonyl group, unsuitability for industrial production, and high cost, so as to achieve reduced electron density and easy operation , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
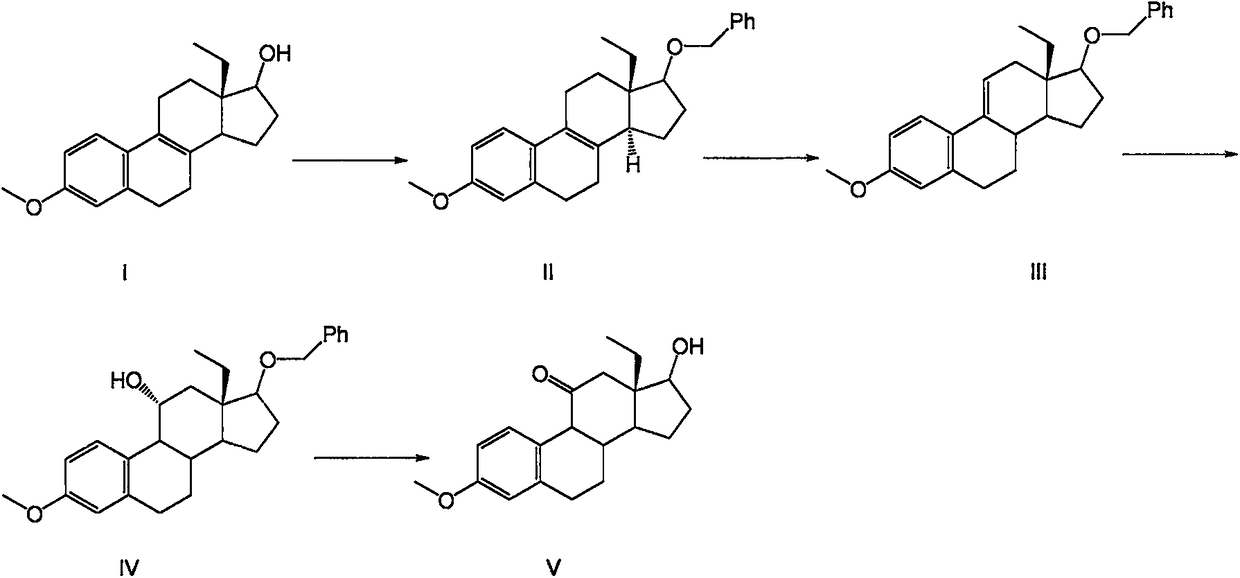
[0049] Embodiment 1: the preparation of compound II (tert-butoxy)
[0050] Add compound I (150g, 0.5mol) into a 3000mL three-neck flask, measure 1500mL of dichloromethane into the three-necked flask, and stir to dissolve. Add 210 mL of pyridine, pivaloyl chloride (120 g, 1 mol) and 1.5 g of DMAP, raise the temperature to 40 ° C, start to reflux, and stir for about 8 hours; after TLC monitors that the reaction is complete, add an appropriate amount of prepared sodium hydroxide solution, adjust the pH to be alkaline, Add 300mL of drinking water and stir, then pour into the separatory funnel, separate the organic phase and add the prepared saturated ammonium chloride aqueous solution, measure the pH of the aqueous phase to 7, separate the organic phase, add the prepared saturated sodium chloride aqueous solution, separate the organic phase Add 20g of anhydrous magnesium sulfate to dry for 30min, filter with suction, and concentrate the filtrate under reduced pressure at P=-0.08Mp...
Embodiment 2
[0052] Embodiment 2: the preparation of compound II (chloro group)
[0053] Take compound I (250g, 0.8mol) and add it into a 5000mL three-necked flask, measure 2500mL of dichloromethane into the three-necked flask, and stir to dissolve. Add 350mL of pyridine, trichloroacetyl chloride (288g, 1.6mol) and 2.5g of DMAP, raise the temperature to 40°C, start to reflux, and stir the reaction for about 8h; after TLC monitors that the reaction is complete, add an appropriate amount of prepared sodium hydroxide solution, and adjust the pH to alkali Add 400mL of drinking water, stir and pour into a separatory funnel, separate the organic phase and add the prepared saturated ammonium chloride aqueous solution, measure the pH of the aqueous phase to 7, separate the organic phase and add the prepared saturated sodium chloride aqueous solution, separate The organic phase was dried by adding 20 g of anhydrous magnesium sulfate for 30 min, filtered with suction, and the filtrate was concentrat...
Embodiment 3
[0055] Embodiment 3: the synthesis of compound III (tert-butoxy)
[0056] The compound (190 g) prepared in Example 1 was weighed and added into a 3000 mL three-necked flask, and 1900 mL of tetrahydrofuran was weighed and added into the three-necked flask, and stirred to dissolve. The reaction flask was evacuated three times, and 290 g of hydrogen peroxide was slowly added into the three-necked flask in batches of 3-5 under the protection of argon, and the temperature was slowly raised to 60°C, and the reaction was stirred for 2-4 hours. After the completion of the reaction was monitored by TLC, the temperature was lowered to 25.±5°C, and the prepared sodium thiosulfate solution was added and stirred for 30 minutes. Pour the reaction solution into a separatory funnel and let it stand, add 400mL ethyl acetate to the aqueous phase to extract once, combine the organic phase and add the prepared sodium chloride aqueous solution to extract once, combine the organic phase, at P=-0.08...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com