A kind of dihydrooxazole antibiotic and preparation method thereof
A technology of dihydrooxazoles and antibiotics, applied in the field of antibiotics, can solve the problem of no antibacterial activity and the like, and achieve the effect of high inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
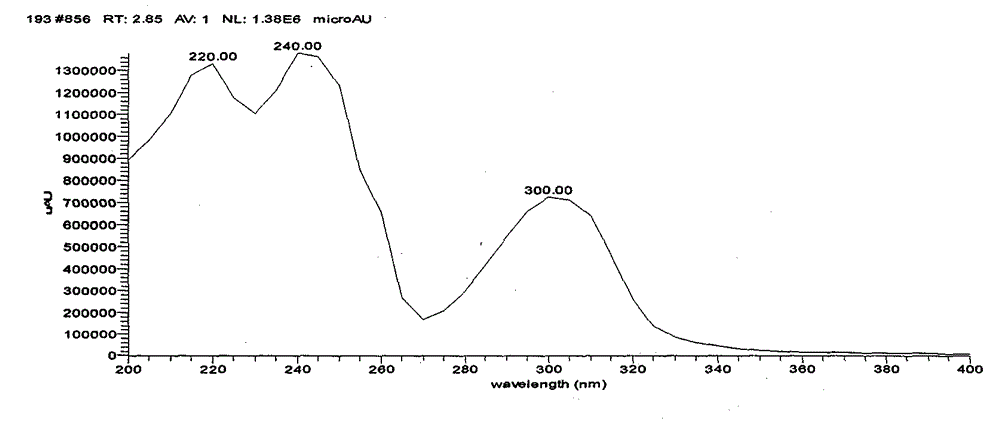
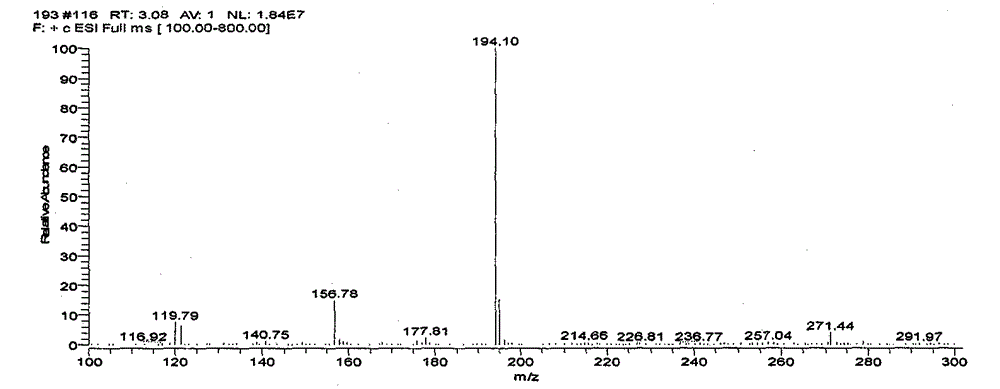
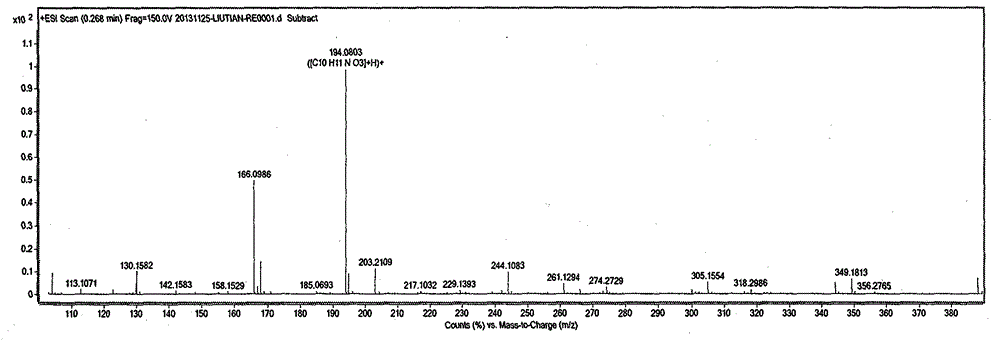
Image
Examples
Embodiment 1
[0042] Fermentation of Antibiotics
[0043] Step 1: Fermentation
[0044] (1) Fermentation strain: The fermentation strain was obtained from soil samples collected from pesticide-contaminated areas in Shandong Peninsula, China, isolated and cultivated in the laboratory, and identified as Streptomyces djakartensis; Deposited by the center, the deposit date is November 13, 2012, and the deposit registration number is CGMCCNo.6817;
[0045] (2) Slant culture: use starch casein culture based on 1×10 5 Sterilize under handkerchief for 30 minutes, culture at 28°C for 5 days after inoculation; the starch casein medium is composed of the following components: 10.0 g of soluble starch, 2.0 g of dipotassium hydrogen phosphate, 2.0 g of potassium nitrate, 2.0 g of sodium chloride, 0.3g casein, 0.05g magnesium sulfate heptahydrate, 0.02g calcium carbonate, 0.01g ferrous sulfate heptahydrate, 20.0g agar, 1000ml distilled water, pH value 7.2;
[0046] (3) Seed culture: subpackage the see...
Embodiment 2
[0066] Antibiotics of chemical synthesis preparation formula (I)
[0067] Add 119mg (1mmol) of o-hydroxybenzocyanide and 30ml of anhydrous methanol to a 50ml dry round bottom flask, dissolve under electromagnetic stirring, add 5ml of acetyl chloride dropwise, stir and react at room temperature for 24h, then add 10ml of triethylamine and 91mg ( 1mmol) serinol, reflux reaction 12h. The reaction solution was poured into 200 ml of ethyl acetate, and washed with 20 ml of water and 20 ml of saturated brine. Dry over anhydrous sodium sulfate and remove the solvent. It was separated by column chromatography and recrystallized from acetone to obtain 116 mg of colorless crystals. The colorless crystalline 1 H-NMR and 13 C-NMR confirms that the structure is an antibiotic shown in formula (I). 1 HNMR (CDCl 3 , 500MHz, δ, ppm): 3.74 (dd, 1H, J = 3.5Hz, 11.5Hz), 3.92 (dd, 1H, J = 3.5Hz, 11.5Hz), 4.34-4.38 (m, 1H), 4.45-4.49 (m, 1H), 4.50-4.52 (m, 1H), 6.84 (1H, m), 6.97 (1H, d, J=8.0...
Embodiment 3
[0069] The bacteriostatic activity of the antibiotic of formula (I) of the present invention
[0070] Adopt microdilution method to measure the antibiotic of formula (I) of the present invention to bacillus cereus (Bacillus cereus), bacillus subtilis (B.subtilis), staphylococcus aureus (Staphylococcus aureus), Escherichia coli (Escherichiacoil), methoxy-resistant The antibacterial activity of 8 kinds of tested bacteria including Staphylococcus aureus (Methicillin-resistant Staphylococcusaureus, MRSA), soft rot fungus of cabbage (Erwinacarotovora), fungus of kiwi fruit canker (Pseudomonassyringae Pv.actinidiae), and Xanthomonasoryzae. Except for methicillin-resistant Staphylococcus aureus, which was provided by Nanjing Medical University, the rest of the strains were purchased from China General Microorganisms Collection Center.
[0071] Pick 4-5 common test colonies with the same shape from the culture plate with an inoculation loop, insert them into Mueller-Hinton broth mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com