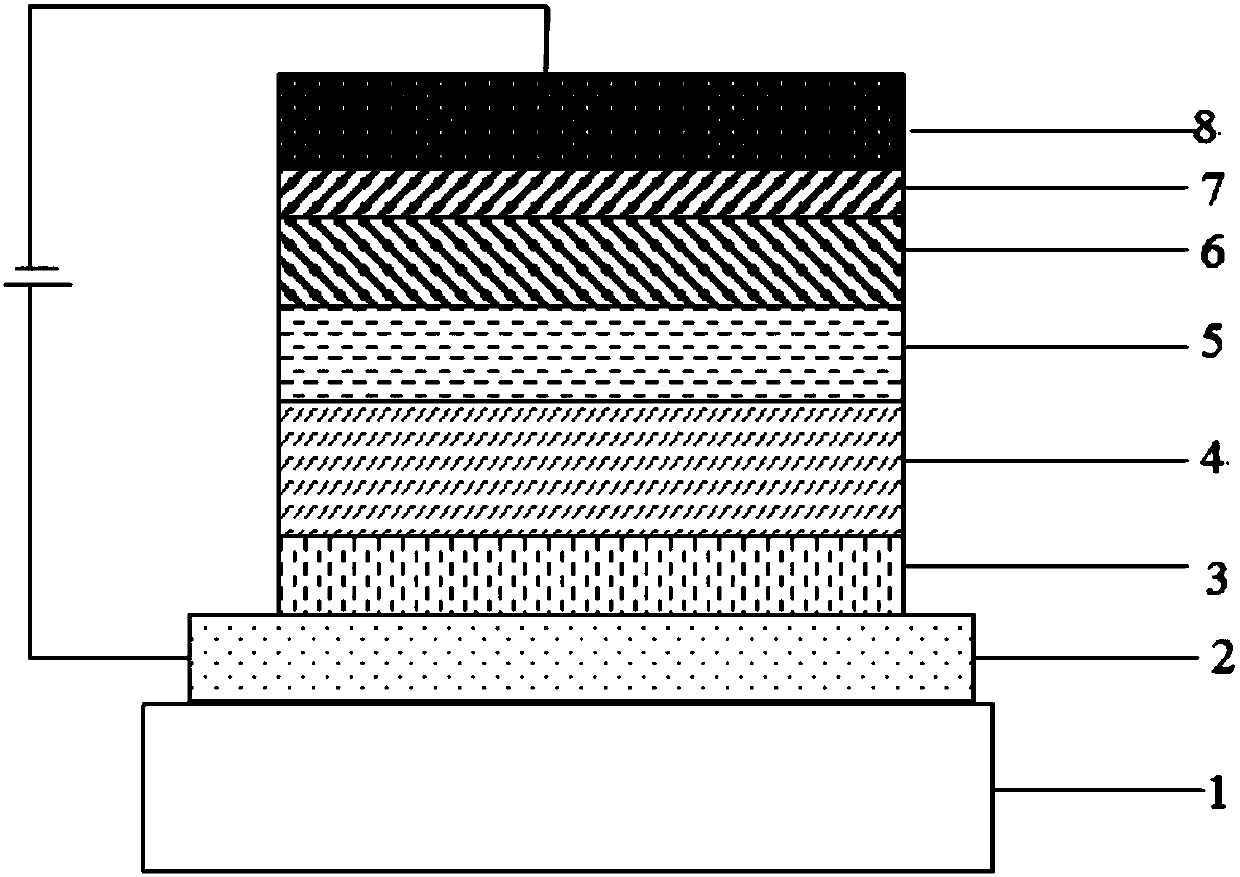
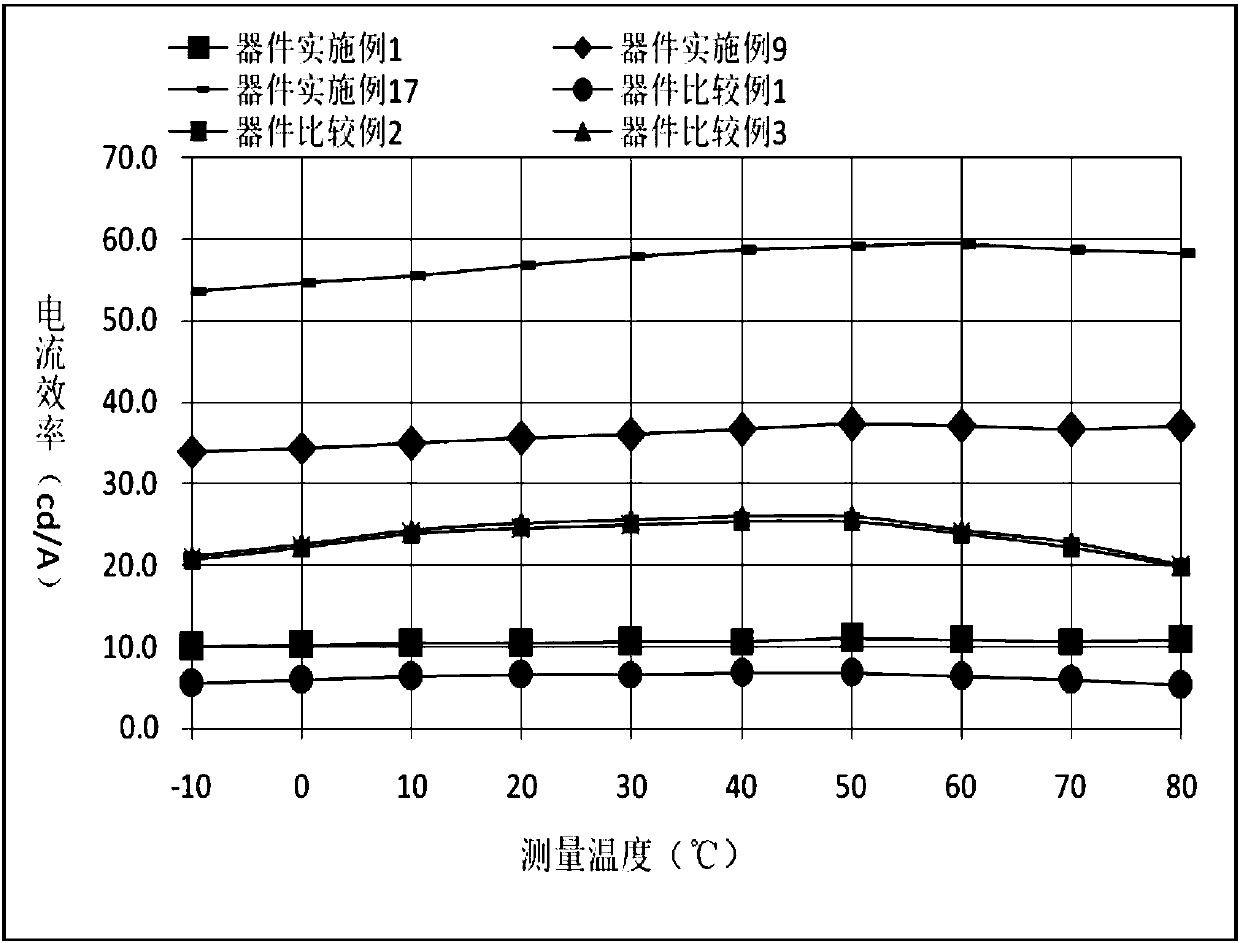
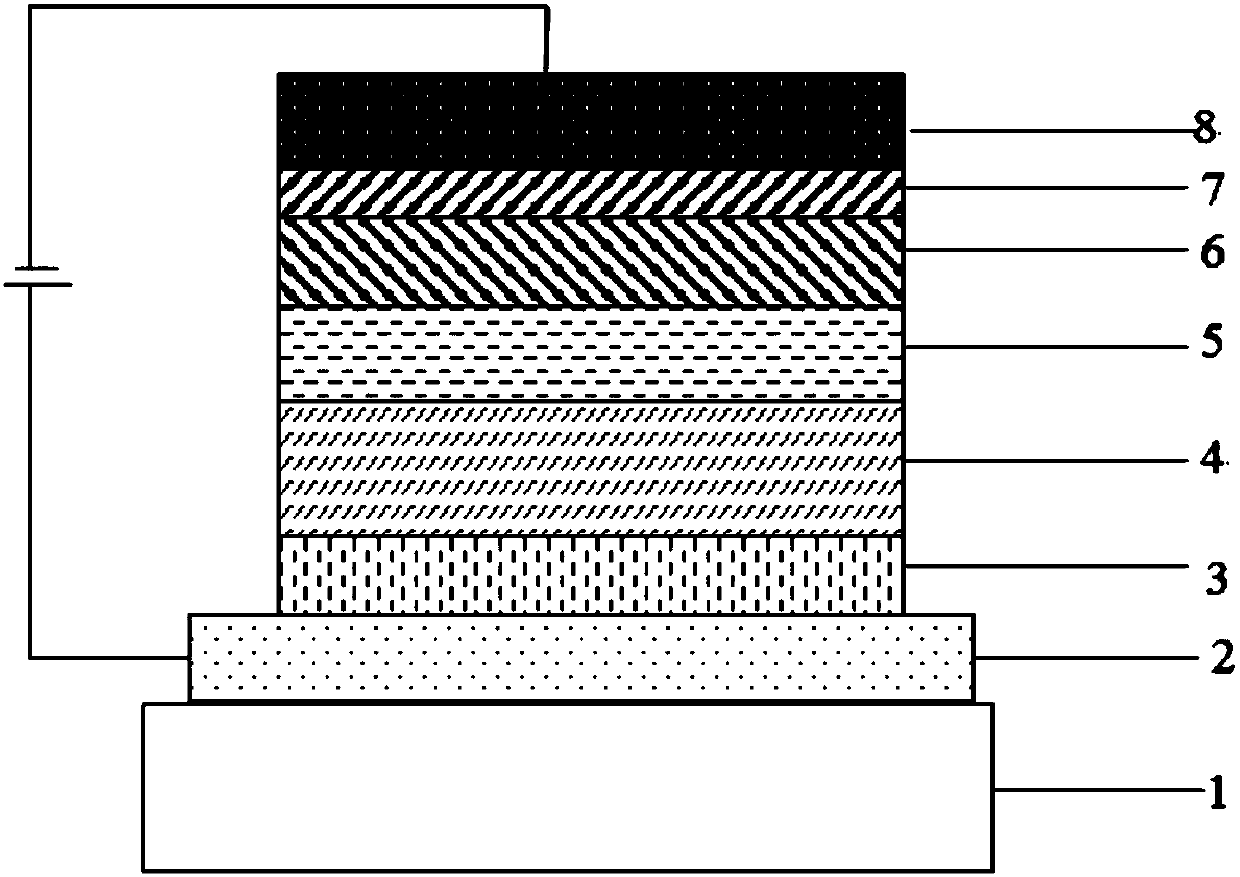
Compound using cyano pyridine as core, and applications in organic electroluminescent devices
A compound, cyano nitrogen technology, applied in the application field of organic electroluminescent devices, can solve the problems of efficiency roll-off, low S1 state radiation transition rate, difficult exciton utilization rate and high fluorescence radiation efficiency, etc., and achieve good The effect of optoelectronic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] First provide the general formula (1) The synthetic route of is:
[0052]
[0053] Weigh the raw material I and dissolve it in acetic acid, cool down to 0°C with an ice-salt bath; weigh the liquid bromine and dissolve it in glacial acetic acid, slowly add it dropwise to the acetic acid solution containing the nitro compound raw material I, and stir at room temperature for 6-12 hours , take a sample point plate, show that there is no raw material I remaining, the reaction is complete, after the reaction, add aqueous sodium hydroxide solution dropwise until the solution is neutral, extract with dichloromethane, get the organic phase and filter, and the filtrate is rotary evaporated under reduced pressure until there is no fraction. Pass through a silica gel column to obtain intermediate S1; in the above reaction, the molar ratio of raw material I to liquid bromine is 1:1 to 3; 30 to 50 mL of acetic acid is used per gram of raw material I;
[0054] Weigh raw material ...
Embodiment 2
[0069] Embodiment 2: the synthesis of compound 7:
[0070]
[0071] Dissolve 0.01mol raw material E-1 and 0.012mol intermediate M1-1 in 150mL anhydrous toluene, add 0.0005mol Pd after deoxygenation 2 (dba) 3 , 0.015mol tri-tert-butylphosphine and 0.02mol sodium tert-butoxide, and reacted at 110°C for 24 hours under an inert atmosphere. During the reaction, the reaction process was continuously monitored by TLC. After the raw materials were completely reacted, cooled and filtered, the filtrate was spun Evaporate and remove solvent, thick product crosses silica gel column, obtains target product; Elemental analysis structure (molecular formula C 46 h 30 N 4 ): theoretical value C, 86.49; H, 4.73; N, 8.77; test value: C, 86.49; H, 4.73; N, 8.78; for 638.66.
Embodiment 3
[0072] Embodiment 3: the synthesis of compound 17:
[0073]
[0074] Dissolve 0.01mol of intermediate M2-2 and 0.012mol of raw material E-1 in 150mL of toluene and ethanol (V 甲苯 :V 乙醇 =5:1) In the mixed solution, add 0.0002mol Pd(PPh 3 ) 4 and 0.02mol K 2 CO 3 , reacted at 110°C for 24 hours under an inert atmosphere. During the reaction process, the reaction process was continuously monitored by TLC. After the raw materials were completely reacted, cooled and filtered, the filtrate was rotary evaporated to remove the solvent, and the crude product was passed through a silica gel column to obtain the intermediate target product ; Elemental analysis structure (molecular formula C 46 h 29 N 3 ): theoretical value C, 88.58; H, 4.69; N, 6.74; test value: C, 88.56; H, 4.69; N, 6.75; for 623.42.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com