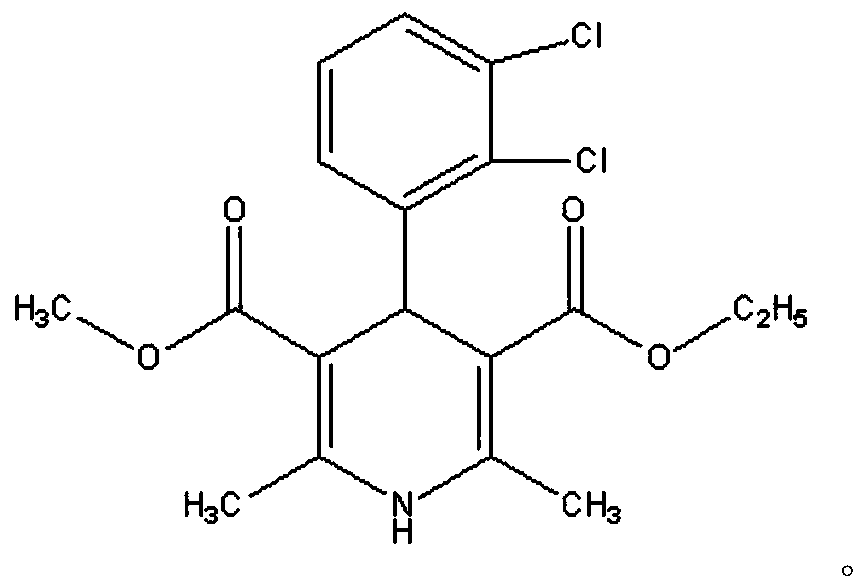
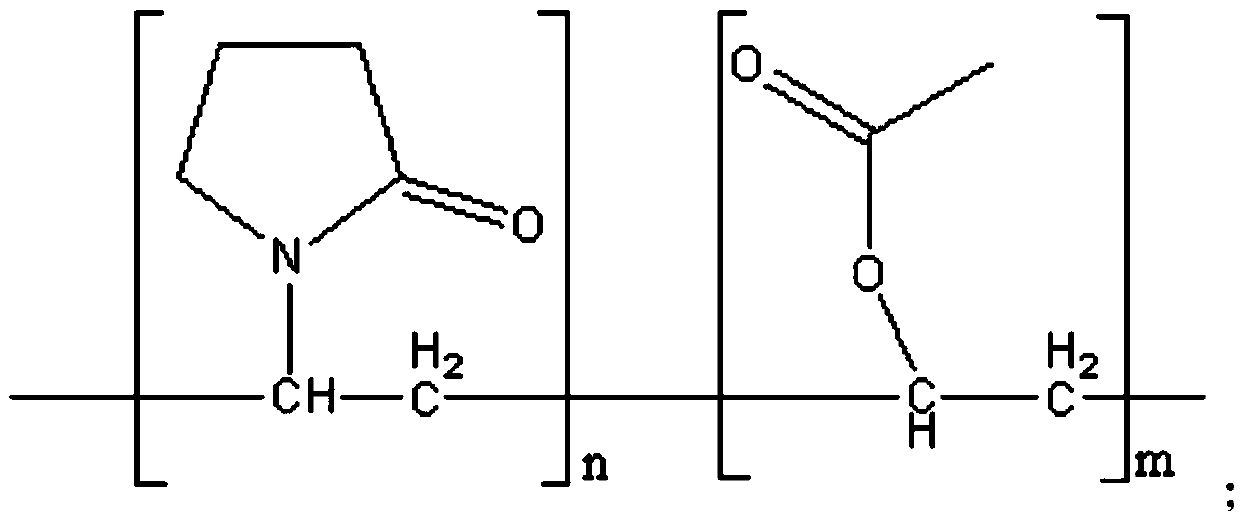
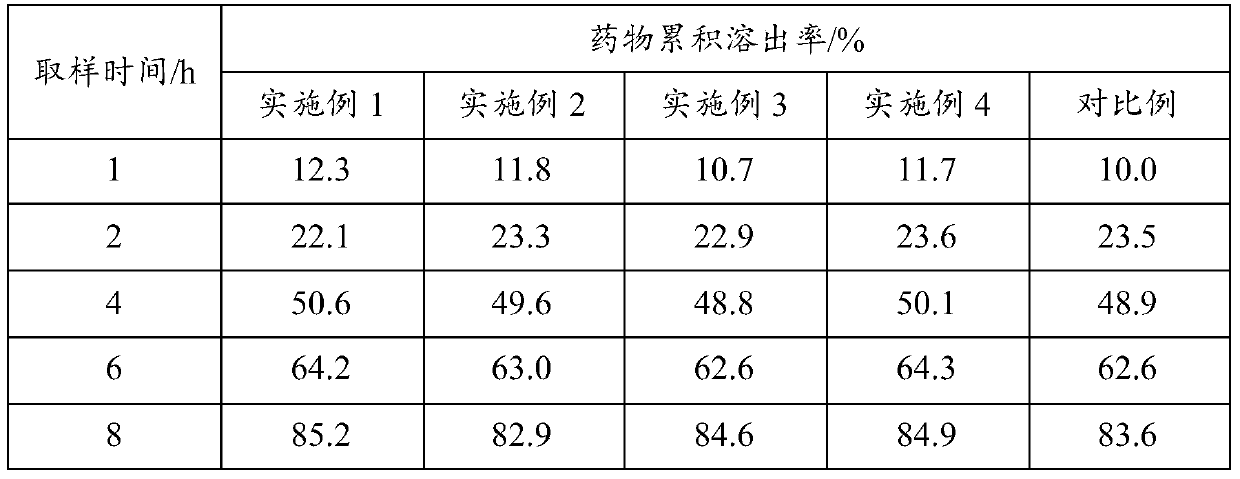
A kind of felodipine sustained-release tablet and preparation method thereof
A gentle and felodipine technology, applied in the field of medicine, can solve the problems of large fluctuation of blood pressure, many times of taking medicine, restricting the development of felodipine sustained-release tablets, etc., and achieves improved uniformity, good sustained-release effect, and stable sustained-release effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of the felodipine sustained-release tablet described in the above technical scheme, comprising the following steps:
[0043] a) After mixing felodipine, dispersion matrix and solvent, cooling and drying are carried out successively to obtain felodipine solid dispersion; the dispersion matrix is copovidone;
[0044] b) compressing the felodipine solid dispersion, filler, lubricant and slow-release material after mixing to obtain a felodipine sustained-release tablet; the slow-release material is hydroxypropyl methylcellulose.
[0045] In the present invention, after mixing the felodipine, the dispersion matrix and the solvent, cooling and drying are carried out in sequence to obtain the felodipine solid dispersion. In the present invention, the felodipine is the main ingredient; the source of the felodipine is not particularly limited in the present invention, and commercially available products well known to tho...
Embodiment 1
[0065](1) Mix 40g copovidone S630 with 200mL absolute ethanol, then add 5g felodipine bulk drug, and heat in a water bath at 70°C for 15min to obtain a dispersion solution of felodipine, then place the above solution on ice Cool in a bath for 12 hours, then dry in a vacuum oven at 50° C. for 36 hours to obtain a solid product, and finally crush the above solid product through an 80-mesh sieve to obtain a solid dispersion of felodipine.
[0066] (2) Felodipine solid dispersion obtained with 60g lactose, 30g microcrystalline cellulose, 2g micropowder silica gel, 4g magnesium stearate, 21g hydroxypropyl methylcellulose K250 and 42g hydroxypropyl methylcellulose K750 is added in the mixer, stirred at 4r / min for 30min, finally adopts tablet press to carry out tabletting, tabletting parameter is φ 9mm, hardness 6kg, obtains felodipine slow-release tablet (1000).
[0067] (3) Adopt 25g Opadry coating powder to carry out coating, obtain felodipine sustained-release tablet product.
Embodiment 2
[0069] (1) Mix 40g of copovidone VA64 with 200mL of absolute ethanol, then add 5g of felodipine crude drug, and heat in a water bath at 70°C for 15min to obtain a dispersion solution of felodipine, then place the above solution on ice Cool in a bath for 10 hours, then dry in a vacuum oven at 55° C. for 30 hours to obtain a solid product, and finally crush the above solid product through an 80-mesh sieve to obtain a solid dispersion of felodipine.
[0070] (2) Felodipine solid dispersion obtained with 60g lactose, 30g microcrystalline cellulose, 2g micropowder silica gel, 4g magnesium stearate, 21g hydroxypropyl methylcellulose K250 and 42g hydroxypropyl methylcellulose K750 is added in the mixer, stirred at 3r / min for 30min, finally adopts tablet press to carry out tabletting, tabletting parameter is φ 9mm, hardness 6kg, obtains felodipine slow-release tablet (1000).
[0071] (3) Adopt 25g Opadry coating powder to carry out coating, obtain felodipine sustained-release tablet p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com