Medical application of CREG protein
A protein and use technology, applied in the field of medical use of CREG protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. Amplification and titer determination of recombinant human CREG adenovirus (AdCREG)
[0039] AdCREG was preserved in the China Type Culture Collection Center (CCTCC, Wuhan, Wuhan University) on January 2, 2008. The name is human adenovirus type 5 Ad5-CREG, and the preservation number is CCTCC-V200801 (see Chinese patent CN101475961A, invention name: Recombinant adenovirus expressing human CREG and use thereof). Incubate 5×10 in DMEM containing 5% fetal bovine serum 6 Ad293 cells (human embryonic kidney cells, purchased from Stratagene, USA, Cat. No. #240085). Take 0.5 μl of AdCREG virus preservation solution, add DMEM containing 5% fetal bovine serum to 1 ml, and mix well to obtain a virus mixture. Remove the culture medium of Ad293 cells, carefully add the virus mixture, and shake slowly 3 times in a cross shape. placed at 37°C CO 2 After incubating in the incubator for 120 min, 9 ml of DMEM culture solution containing 5% fetal bovine serum was added,...
Embodiment 2
[0043] Example 2. Preparation of atherosclerosis mouse model
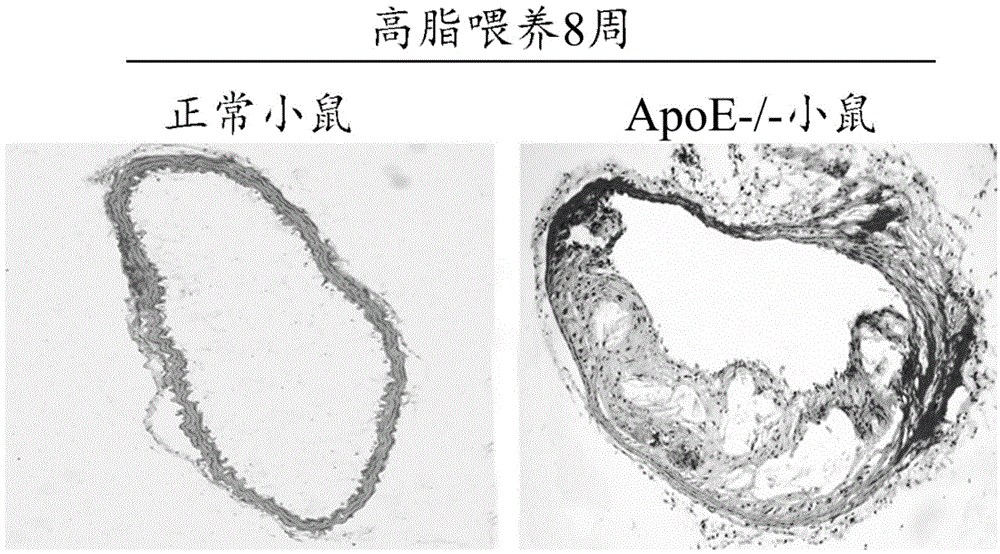
[0044] 8-week-old ApoE gene knockout (ApoE - / - ) mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), were given high-fat feeding after weaning. High-fat feed formula: 15% fat, 0.25% cholesterol, 84.75% common feed. The mouse model of atherosclerosis was prepared after continuous feeding for 8 weeks. The experimental results showed that compared with the blood vessels of normal mice, apoE- / - mice could see obvious atherosclerotic plaque protruding into the lumen after 8 weeks of high-fat feeding. Visible lipid vacuoles, cholesterol crystals, a large number of inflammatory cell infiltration ( figure 2 ). The above results indicated that the mouse model of atherosclerosis was successfully prepared.
Embodiment 3
[0045] Example 3. Tail vein injection of adenovirus for the treatment of atherosclerosis
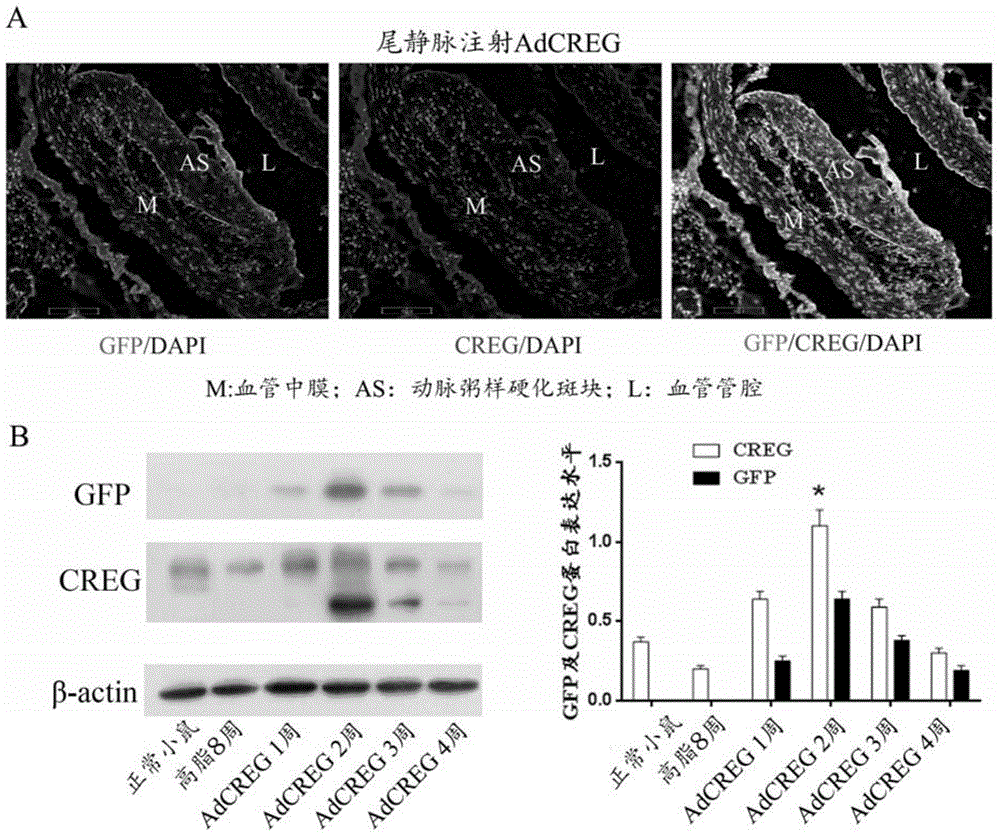
[0046] The ApoE- / - mice with atherosclerosis were randomly divided into AdCREG group (n=20), AdGFP group (n=20) and normal saline control group (n=20) after continuous high-fat feeding for 8 weeks. The number of mice for each index is 3-5. In the AdCREG group, the titer was 4.75×10 by tail vein injection. 10 1 ml of saline containing 1 viral plaque forming unit (PFU) of AdCREG. In the AdGFP group, the titer was 4.75×10 by tail vein injection. 10 PFU of AdGFP in 1ml of saline. The normal saline control group was injected with 1ml of physiological saline through the tail vein. After continuing to feed for 8 weeks, the mice were sacrificed by neck dislocation, and the aorta blood vessels were taken.
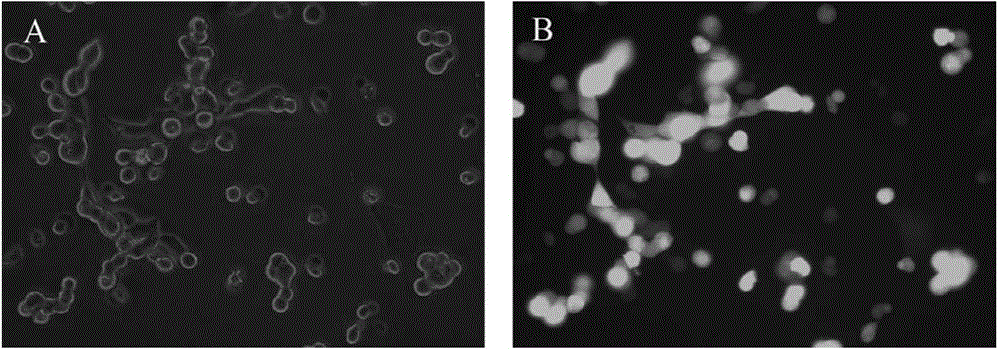
[0047] For the blood vessels that have been taken, the infection efficiency of the recombinant adenovirus was identified. In the present invention, the recombinant adenovirus AdGFP has a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com