Preparation method of hollow sulfide nanometer material
A nanomaterial and sulfide technology, applied in the field of preparation of hollow sulfide nanomaterials, achieves the effects of uniform morphology, large specific surface area and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
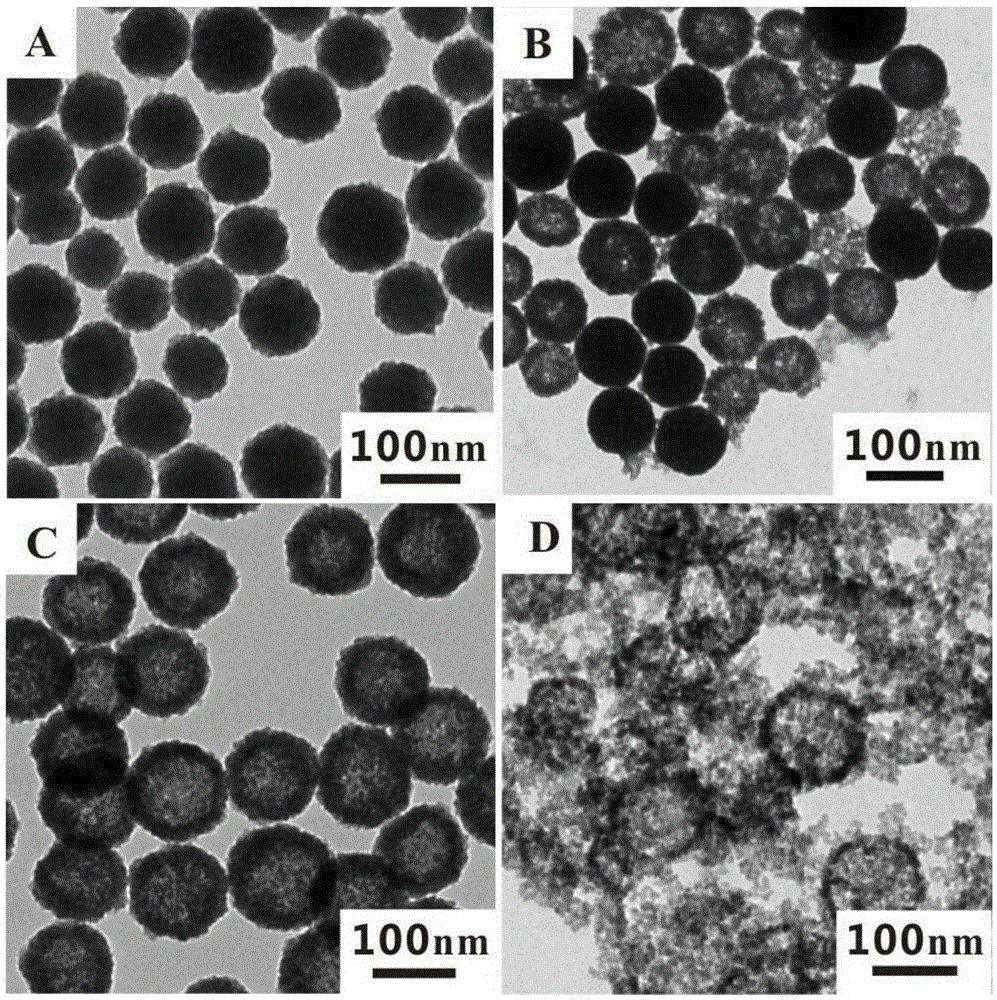
[0026] 1) Preparation of solid zinc sulfide nanomaterials
[0027] According to the existing literature (XiaoxiaoYu, JiaguoYu, BeiCheng, BaibiaoHuang, One-PotTemplate-FreeSynthesis of MonodisperseZincSulfideHollowSpheres and TheirPhotocatalyticProperties.Chemistry-AEuropeanJournal, 2009, 15, 6731-6739), 0.8mmol of zinc acetate and 20mmol of thiourea were added to 20mL of water Stir at room temperature for half an hour, then transfer to a reaction kettle, react at 140°C for 50 minutes, centrifuge and wash the reacted product, and finally obtain about 6 mg of the product, disperse the obtained product in 6 mL of ethanol, and store Reserve at 4°C.
[0028] 2) Preparation of hollow zinc sulfide nanomaterials
[0029] Get 1mL of the above-mentioned ethanol solution dispersed with solid zinc sulfide nanomaterials and add it to a 25mL round bottom flask, then dilute with 4mL of ethanol, the weight ratio of the solid zinc sulfide nanomaterials and ethanol is about 1:4000, Add 4 mL, ...
Embodiment 2
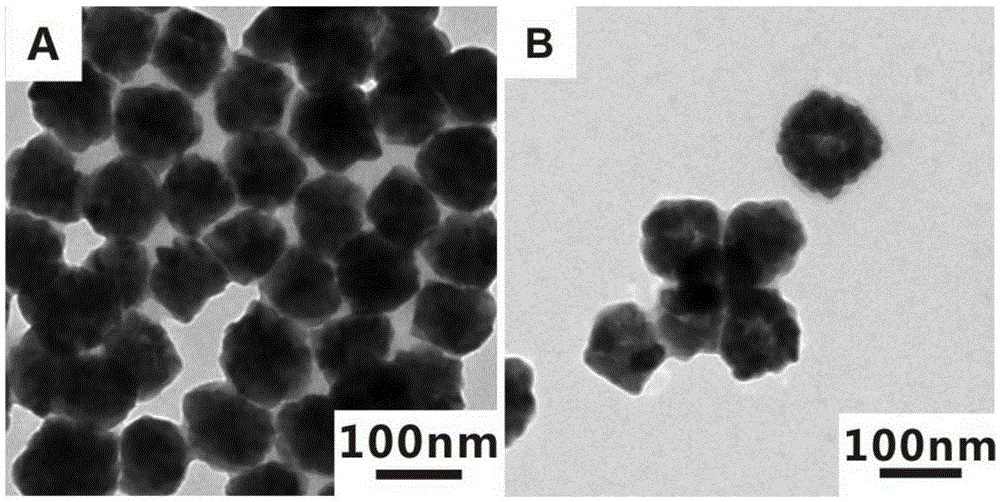
[0031] 1) Preparation of solid zinc sulfide nanomaterials
[0032]According to the existing literature (XiaoxiaoYu, JiaguoYu, BeiCheng, BaibiaoHuang, One-PotTemplate-FreeSynthesis of MonodisperseZincSulfideHollowSpheres and TheirPhotocatalyticProperties.Chemistry-AEuropeanJournal, 2009, 15, 6731-6739), 0.8mmol of zinc acetate and 20mmol of thiourea were added to 20mL of water Stir at room temperature for half an hour, then transfer to a reaction kettle, react at 140°C for 50 minutes, centrifuge and wash the reacted product, and finally obtain about 6 mg of the product, disperse the obtained product in 6 mL of ethanol, and store Reserve at 4°C.
[0033] 2) Preparation of hollow zinc sulfide nanomaterials
[0034] Get 1mL of the above-mentioned ethanol solution dispersed with solid zinc sulfide nanomaterials and add it to a 25mL round bottom flask, then dilute with 4mL of ethanol, the weight ratio of the solid zinc sulfide nanomaterials and ethanol is about 1:4000, Add 4 mL, 4...
Embodiment 3
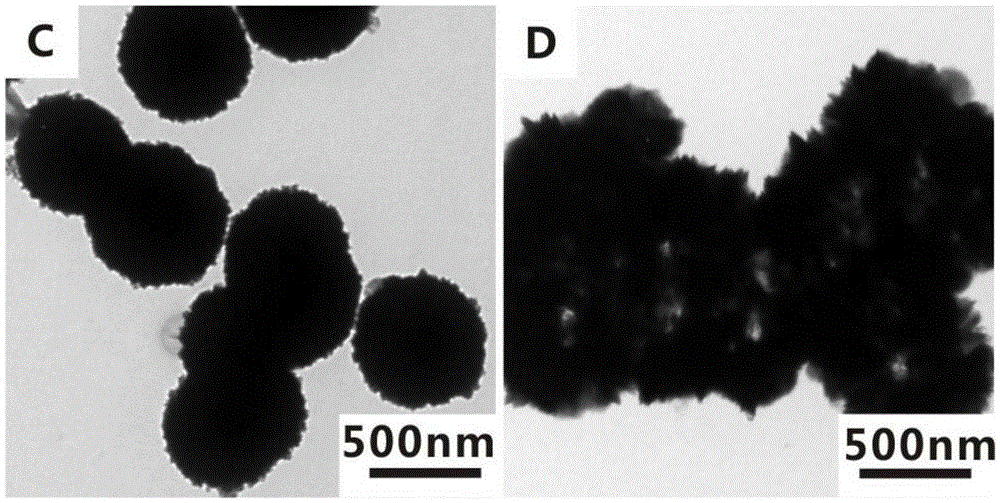
[0036] 1) Preparation of solid zinc sulfide nanomaterials
[0037] According to the existing literature (XiaoxiaoYu, JiaguoYu, BeiCheng, BaibiaoHuang, One-PotTemplate-FreeSynthesis of MonodisperseZincSulfideHollowSpheres and TheirPhotocatalyticProperties.Chemistry-AEuropeanJournal, 2009, 15, 6731-6739), 0.8mmol of zinc acetate and 20mmol of thiourea were added to 20mL of water Stir at room temperature for half an hour, then transfer to a reaction kettle, react at 140°C for 50 minutes, centrifuge and wash the reacted product, and finally obtain about 6 mg of the product, disperse the obtained product in 6 mL of ethanol, and store Reserve at 4°C.
[0038] 2) Preparation of hollow zinc sulfide nanomaterials
[0039] Get 1mL of the above-mentioned ethanol solution dispersed with solid zinc sulfide nanomaterials and add it to a 25mL round bottom flask, then dilute with 4mL of ethanol, the weight ratio of the solid zinc sulfide nanomaterials and ethanol is about 1:4000, Add 4 mL, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap