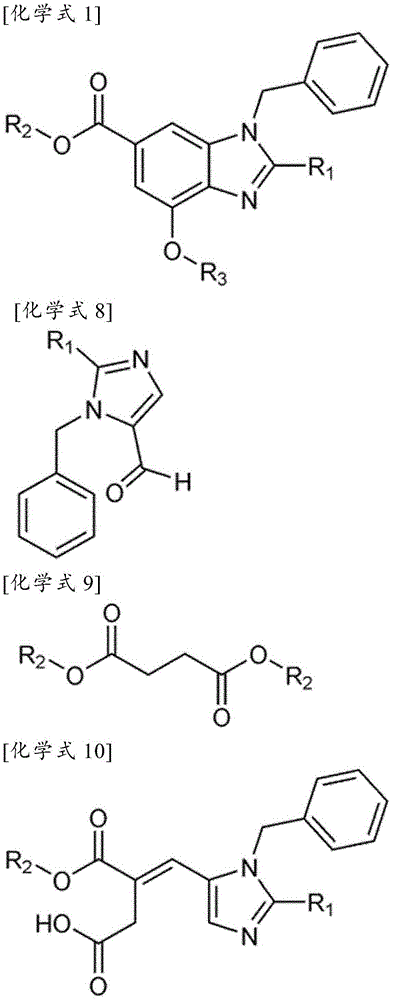
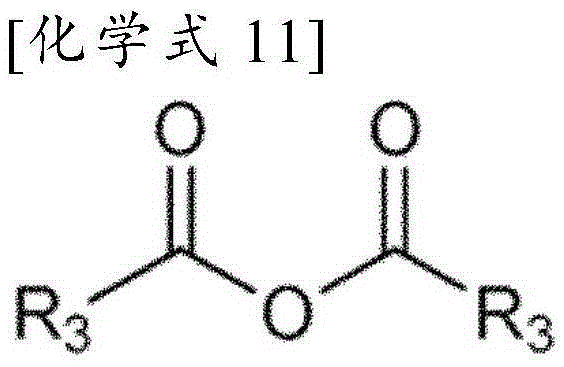
Method for preparation of benzimidazole derivatives
一种苯并咪唑、衍生物的技术,应用在制备苯并咪唑衍生物领域,能够解决暴露等问题,达到优良制造收率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: step a
Embodiment 1-1
[0090] 1) embodiment 1-1: the preparation of acetimidic acid methyl ester hydrochloride
[0091] 1.6 kg of acetonitrile and 2.4 kg of methanol were added to the reactor and the reactor was cooled to -10°C. Acetyl chloride in an amount of 3.67 kg was slowly added thereto and the mixture was stirred at 0°C for 12 hours. While stirring, the internal temperature of the reactor was maintained at 0°C. At the end of the reaction, the solvent was removed under reduced pressure at 45°C. 11.8 kg of tert-butyl methyl ether were added to the reaction mixture and the mixture was stirred at 0°C for 3 hours. The resulting solid was filtered and vacuum-dried at 40° C. to obtain 4.1 kg of methyl acetimidic acid hydrochloride (yield: 95%).
[0092] 1 H-NMR (400MHz, DMSO-d 6 ): 11.7(s, 2H), 4.07(s, 3H), 2.38(s, 3H)
Embodiment 1-2
[0093] 2) embodiment 1-2: the preparation of acetimidic acid ethyl ester hydrochloride
[0094] 1.6 kg of acetonitrile and 4.3 kg of ethanol were added to the reactor and the reactor was cooled to -10°C. Acetyl chloride in an amount of 3.67 kg was slowly added thereto and the mixture was stirred at 0°C for 12 hours. While stirring, the internal temperature of the reactor was maintained at 0°C. At the end of the reaction, the solvent was removed under reduced pressure at 45°C. 11.8 kg of tert-butyl methyl ether were added to the reaction mixture and the mixture was stirred at 0°C for 3 hours. The resulting solid was filtered and vacuum-dried at 40° C. to obtain 4.58 kg of ethyl acetimidate hydrochloride (yield: 95%).
[0095] 1 H-NMR (400MHz, DMSO-d 6 ): 11.7(s, 2H), 4.26(q, 2H), 2.38(s, 3H), 1.36(t, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com