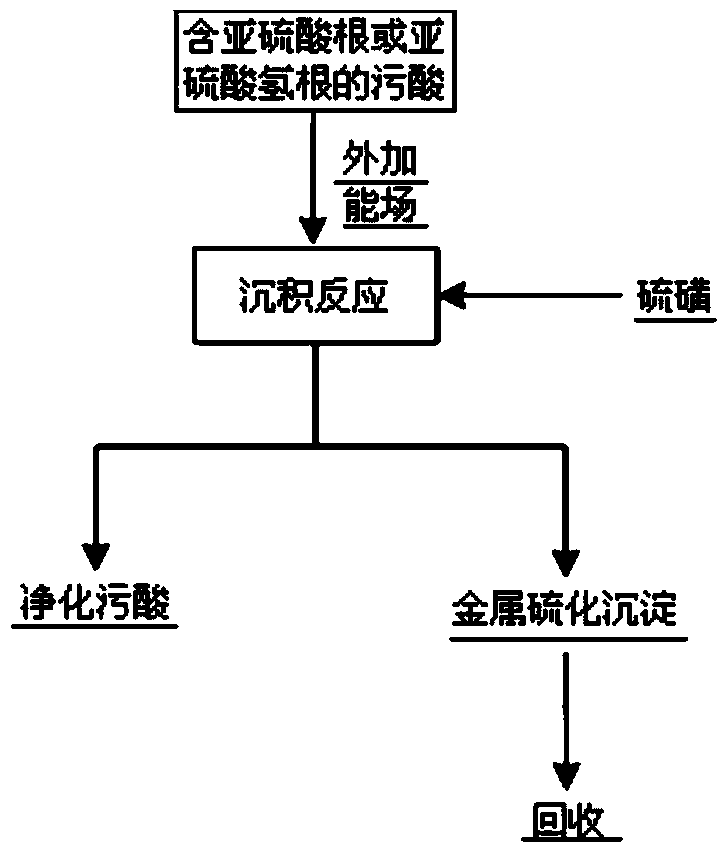
A method for efficiently removing heavy metals in polluted acid
A technology of heavy metals and heavy metal ions, which is applied in the field of comprehensive utilization of waste resources, can solve the problems of high cost and large amount of lye, and achieve the effects of low cost, easy acquisition and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
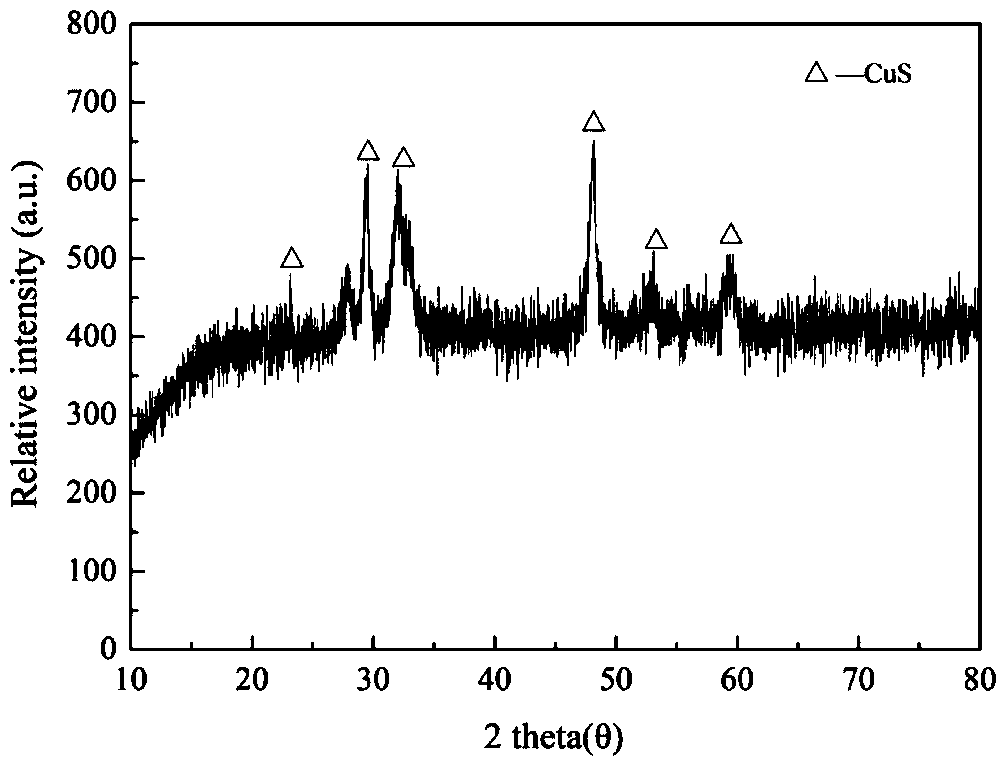
[0037] Using Cu(NO 3 ) 2 、Na 2 SO 3 and H 2 SO 4 Formulated with Cu 2+ The concentration is 2.00g / L, containing SO 3 2- The concentration is 0.2g / L, containing SO 4 2- 100mL simulated dirty acid solution with a concentration of 75g / L. Bubble 3% simulated SO into the solution 2 Flue gas into the simulated sewage acid solution, the flow rate is 0.3L / min, the initial SO 2 The removal rate is 54%. After about 60 minutes of feeding, the sulfite and bisulfite ions contained in the dirty acid can meet the requirements for precipitation of heavy metals; Black precipitate. The precipitate was removed by filtration, and the XRD characterization result was figure 2 Shown; Get filtrate and carry out atomic absorption analysis, the content of copper ion is 0.63g / L, and copper ion removal rate is 68.50%.
Embodiment 2
[0039] This case simulates a high-concentration SO 3 2- of polluted acid, using Cu(NO 3 ) 2 、Na 2 SO 3 and H 2 SO4 Formulated with Cu 2+ The concentration is 2.00g / L, containing SO 3 2- The concentration is 74g / L, containing SO 4 2- 100mL simulated dirty acid solution with a concentration of 75g / L, and the sulfite and bisulfite ions contained in the dirty acid meet the requirements for precipitation of heavy metals; transfer the above solution to a reaction kettle, heat it to 160°C for 2 hours, and produce a large amount of Black precipitate. The precipitate was removed by filtration, and the result of XRD characterization was CuS; the filtrate was taken for atomic absorption analysis, the content of copper ions was 0.35g / L, and the removal rate of copper ions was 82.50%.
Embodiment 3
[0041] This case simulates a high-concentration SO 3 2- of polluted acid, using Cu(NO 3 ) 2 、Na 2 SO 3 and H 2 SO 4 Formulated with Cu 2+ The concentration is 2.00g / L, containing SO 3 2- The concentration is 74g / L, containing SO 4 2- 100mL simulated dirty acid solution with a concentration of 81g / L, and the sulfite and bisulfite ions contained in the dirty acid meet the requirements for precipitation of heavy metals; then add 0.20g sublimated sulfur to the solution, and stir to mix. The above solution was transferred to a reaction kettle, heated to 100° C. for 2 h, and a large amount of black precipitates were produced. The precipitate was removed by filtration, and the result of XRD characterization was CuS; the filtrate was taken for atomic absorption analysis, and the content of copper ions was 0.61×10 -3 g / L, the removal rate of copper ions is 99.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com