Preparing method for ambroxol salbutamol lipid solid dispersion
A technology of ambroxolbuterol and solid dispersion, applied in the field of ambroxol salbutamol lipid solid dispersion and preparation thereof, can solve the problems such as no reports of lipid solid dispersion, and achieves the treatment of respiratory system diseases and stable quality , the effect is remarkable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
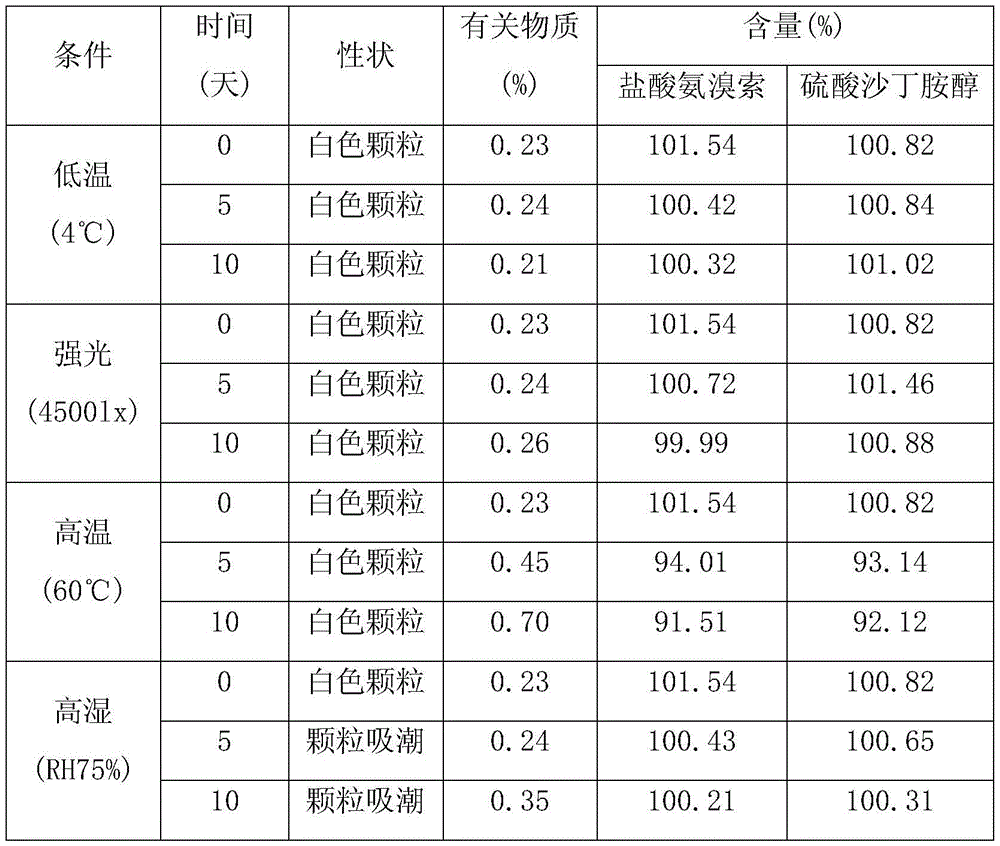
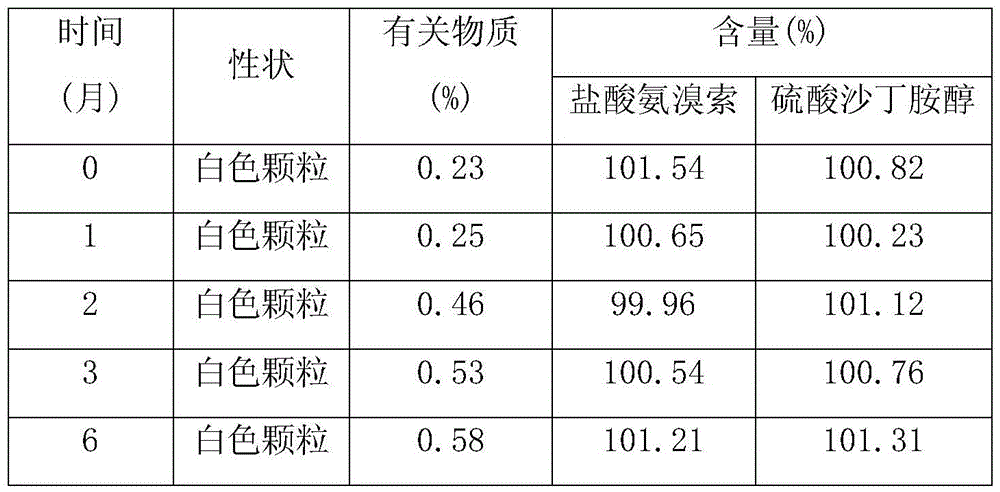
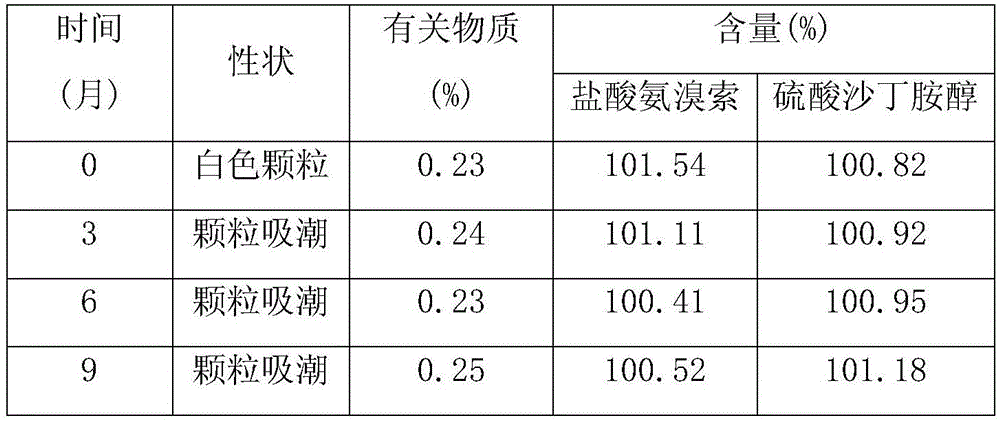
Image
Examples
Embodiment 1
[0020] Mix 25% glyceryl trilaurate, 30% coconut oil, 15% polyethylene glycol caprylic acid / capric glyceride, 20% vitamin E acetate and 10% PEG200 to obtain a lipid carrier, 30% micropowder silica gel and Mix 70% cornstarch uniformly to obtain a solid carrier, pulverize 15g of ambroxol hydrochloride and 20g of salbutamol sulfate through a 120 mesh sieve, add 5g of butylated hydroxyanisole and mix uniformly to obtain a drug mixture, and the drug mixture is in a mass ratio of 1:1000 Add to the molten lipid carrier, vortex, ultrasonic for 30min to dissolve evenly, to obtain the lipid mixture, add the lipid mixture in the molten state to the solid carrier whose mass is 1:2 with the lipid carrier to absorb and solidify .
Embodiment 2
[0022] Mix 25% glyceryl trilaurate, 30% coconut oil, 15% polyethylene glycol caprylic acid / capric glyceride, 20% vitamin E acetate and 10% PEG200 to obtain a lipid carrier, 30% micropowder silica gel and Mix 70% cornstarch uniformly to obtain a solid carrier, pulverize 15g of ambroxol hydrochloride and 20g of salbutamol sulfate through a 120 mesh sieve, add 5g of butylated hydroxyanisole and mix uniformly to obtain a drug mixture, and the drug mixture is in a mass ratio of 1:1500 Add to the molten lipid carrier, vortex and ultrasonic for 30 minutes to dissolve evenly to obtain a lipid mixture, add the lipid mixture in a molten state to a solid carrier with a mass of lipid carrier of 1:3 to absorb and solidify .
Embodiment 3
[0024] Mix 25% glyceryl trilaurate, 30% coconut oil, 15% polyethylene glycol caprylic acid / capric glyceride, 20% vitamin E acetate and 10% PEG200 to obtain a lipid carrier, 30% micropowder silica gel and Mix 70% cornstarch uniformly to obtain a solid carrier, pulverize 15g of ambroxol hydrochloride and 20g of salbutamol sulfate through a 120 mesh sieve, add 5g of butylated hydroxyanisole and mix uniformly to obtain a drug mixture, and the drug mixture is in a mass ratio of 1:2000 Add to the molten lipid carrier, vortex and ultrasonic for 30 minutes to dissolve evenly to obtain a lipid mixture, add the lipid mixture in a molten state to a solid carrier with a mass ratio of 1:4 to the lipid carrier to absorb and solidify .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com