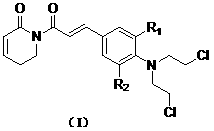
Application of Nitrogen mustardyl perylene amide compounds in medicine
A kind of technology of perylene amide and compound, which is applied in the field of application of nitrogen mustard-based peronyl amide compounds in medicine, and can solve problems such as the application of nitrogen mustard-yl perylene amide compounds that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
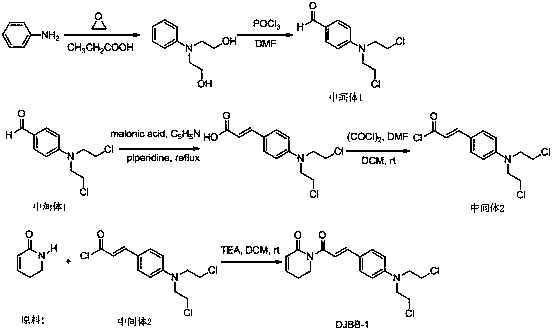
[0017] Example 1: Compound DJBB-1 preparation of
[0018] The synthetic route is as follows:
[0019]
[0020] For the synthetic route of intermediate 1, please refer to the reference (Liu Wenhu et al., Acta Pharmaceutica Sinica, 2014, 49 (2): 217 −224).
[0021] The synthetic route of intermediate 2 can be found in reference (Shoujiao Peng et al, J. Med. Chem. 2015,58, 5242−5255).
[0022] The synthetic route of compound DJBB-1 can be found in the reference (Shoujiao Peng et al, J. Med. Chem.2015, 58, 5242−5255):
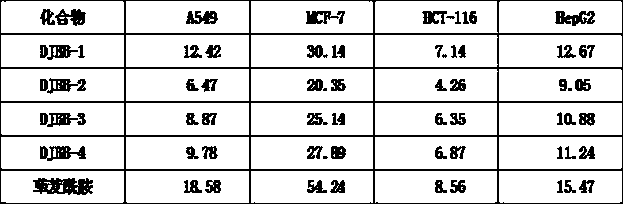
[0023] Add raw material 1 10 mmol, intermediate 2 10 mmol, CH 2 Cl 2 10 ml, 10 ml of triethylamine, stirred at room temperature for 10 h. Then add saturated NH 4 Washed with Cl solution, CH 2 Cl 2 Extraction, washing with saturated brine, MgSO 4 Dried and concentrated. The crude product was separated by column chromatography with a yield of 65%.
[0024] 1 H NMR (400 MHz, CDCl 3 ): δ7.89 (d, J = 15.6 Hz,1H), 7.60-7.54 (m, 2H),7.34 (d, J = 15.6 Hz...
Embodiment 2
[0026] Example 2: Compound DJBB-2 preparation of
[0027] The synthetic route is the same as compound DJBB-1.
[0028] The yield of compound DJBB-2 was 60%.
[0029] 1 H NMR (400 MHz, CDCl 3 ): δ7.90 (d, J = 15.6 Hz,1H),7.32 (d, J = 15.6Hz,1H), 6.96-6.92 (m, 3H), 6.02 (t, J = 9.6 Hz,1H), 4.06 (t, J = 6.4 Hz, 2H), 3.89 (s, 6H), 3.86 (t, J = 7.6 Hz, 4H), 3.74 (t, J = 7.6 Hz, 4H), 2.42 (m, 2H).
[0030] MS-ESI (m / z): 449.10 (M+Na 十 ).
Embodiment 3
[0031] Example 3: Compound DJBB-3 preparation of
[0032] The synthetic route is the same as compound DJBB-1.
[0033] The yield of compound DJBB-3 was 56%.
[0034] 1 H NMR (400 MHz, CDCl 3 ): δ7.92 (d, J = 15.6 Hz,1H),7.36 (d, J = 15.6Hz,1H), 6.96-6.92 (m, 3H), 6.03 (t, J = 9.6 Hz,1H), 4.03 (t, J = 6.4 Hz, 2H), 3.82 (t, J = 7.6 Hz, 4H), 3.76 (t, J = 7.6 Hz, 4H), 2.46 (m, 2H).
[0035] MS-ESI (m / z): 425.06 (M+Na 十 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com