Macromolecular photoinitiator containing diphenyl ketone groups and preparation method of macromolecular photoinitiator
A technology of photoinitiator and benzophenone, which is applied in the field of macromolecular photoinitiator and its preparation, and can solve problems such as toxicity and product odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
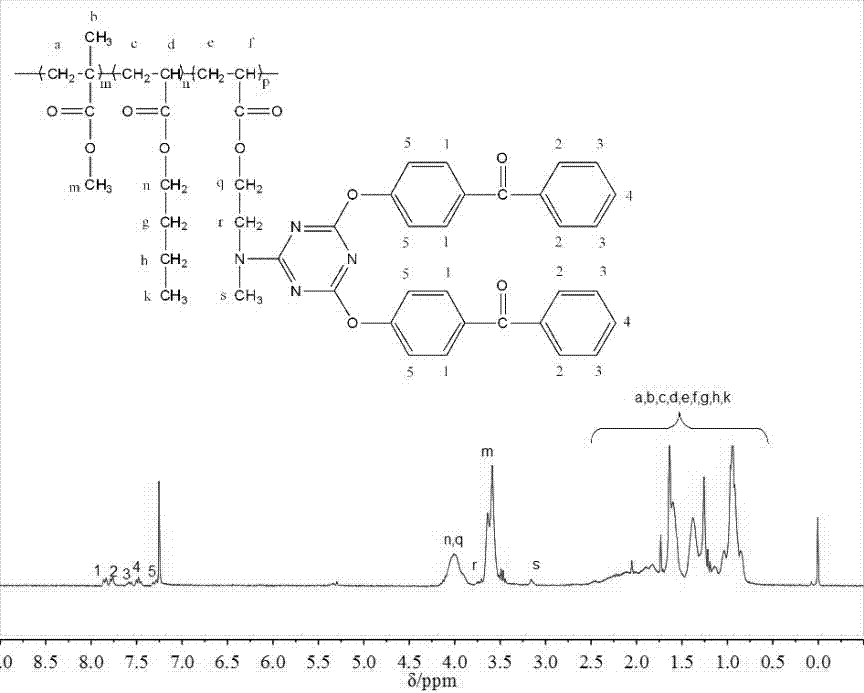
[0067] The present embodiment provides a kind of macroinitiator ADBPT-10, is prepared according to the following steps:
[0068] S1. Synthesis of triazine derivatives containing two benzophenone units:
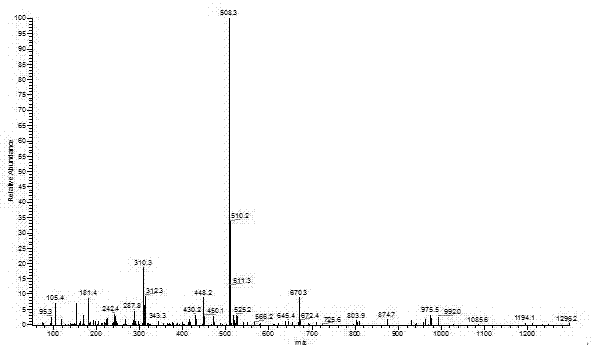
[0069] Add 4.66g (25mmol) of cyanuric chloride and 0.41g (1.25mmol) of tetrabutylammonium bromide to a 500mL single-necked flask, dissolve them in 150mL of dichloromethane, and place them in a low-temperature tank at 0-5°C. Another 10.50g (52.5mmol) of 4-hydroxybenzophenone and 2.10g (52.5mmol) of NaOH were taken in a 150mL beaker, dissolved in 150mL of distilled water, and added dropwise to the dichloromethane solution. After the dropwise addition, the temperature was raised to about 12°C, and the reaction was carried out at a constant temperature for 12 hours to obtain a colorless liquid with two phases, which was separated with a separatory funnel. The organic phase was extracted 3-4 times with distilled water, anhydrous MgSO 4 After drying, filtering and rotary evaporati...
Embodiment 2
[0082] S1, S2, S3 are the same as the corresponding steps in Embodiment 1.
[0083] S4. Synthesis of macromolecular photoinitiator:
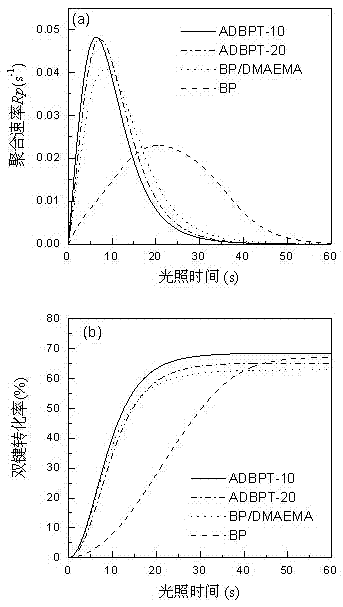
[0084] In this embodiment, the amount of the polymerizable photoinitiator ADBPT is increased to 20% by mass, and the rest are the same as in Embodiment 1. The resulting macromolecular photoinitiator is designated as ADBPT-20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com