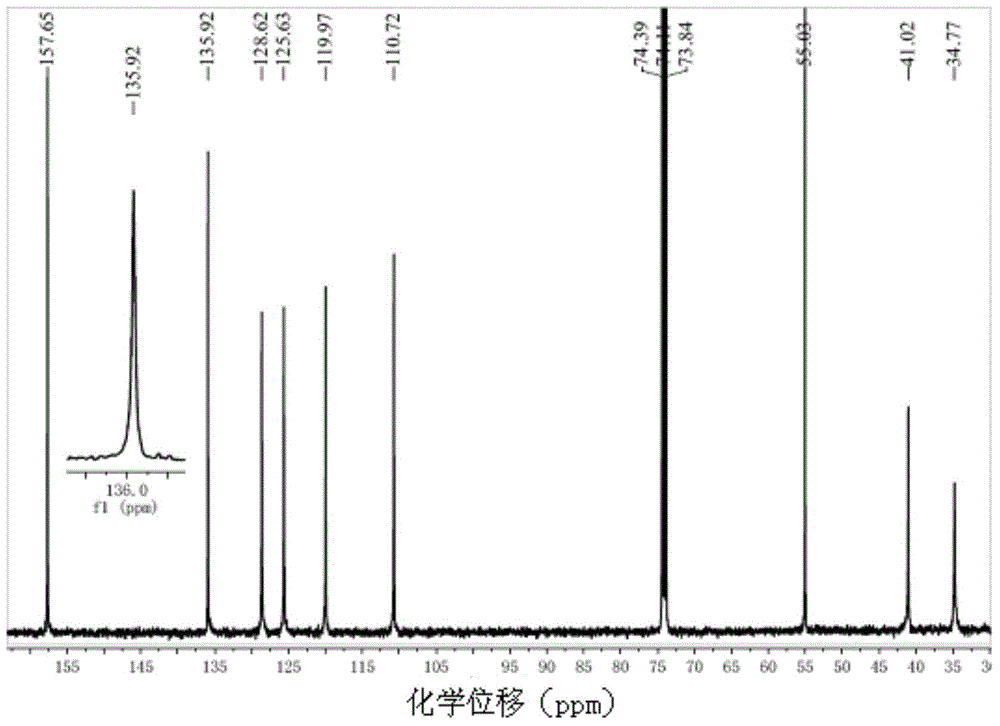
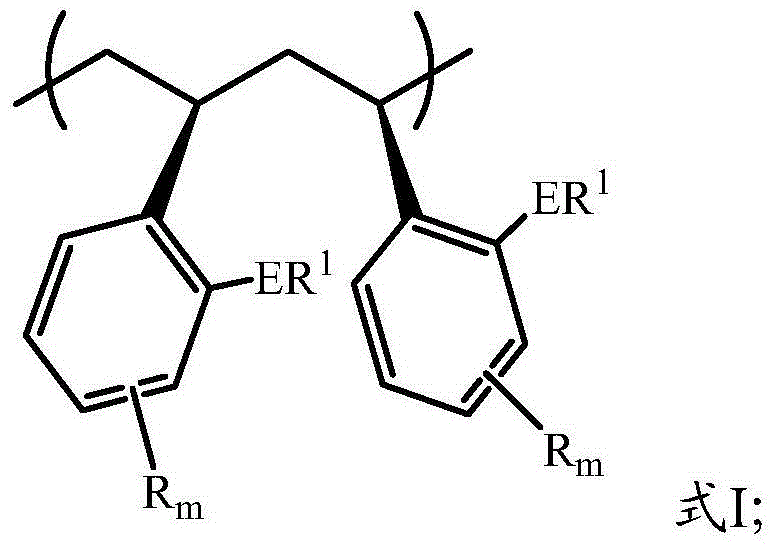
Functional highly-isotactic polystyrene and preparation method thereof
A technology of isotactic polystyrene and styrene, applied in the field of polymers, can solve the problems of low selectivity, loss of activity, inability to obtain high isotactic polystyrene, etc., and achieve high isotactic selectivity and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of the functionalized high isotactic polystyrene provided by the present invention comprises: performing a polymerization reaction on the functionalized styrene under the action of a catalyst to obtain the functionalized high isotactic polystyrene;
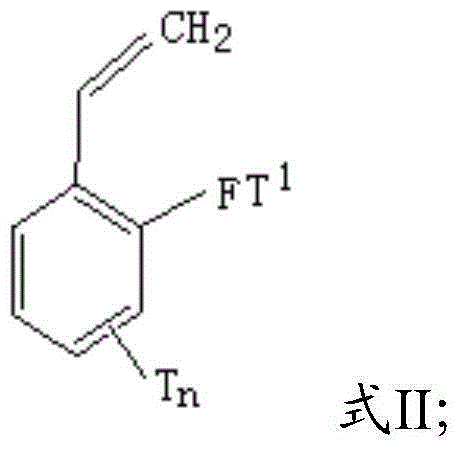
[0057] The functionalized styrene has a structure shown in formula II:
[0058]
[0059] In formula II, F is sulfur or oxygen;
[0060] T 1 An alkyl group with 1 to 10 carbon atoms or an aryl group with 6 to 10 carbon atoms;
[0061] T is an alkoxy group with 1 to 20 carbon atoms, an aryloxy group with 6 to 20 carbon atoms, an alkylthio group with 1 to 20 carbon atoms, an arylthio group with 6 to 20 carbon atoms, An alkyl group with 1 to 20 carbon atoms or an aryl group with 6 to 20 carbon atoms; T is substituted at any position on the benzene ring;
[0062] n represents the number of substituent T, 0≤n≤4;
[0063] The catalysts include rare earth complexes, organoboron compounds and organoalum...
Embodiment 1
[0118] Under anhydrous and oxygen-free conditions, 5mL of tetrahydrofuran solution with a concentration of 0.1mol / L having a ligand shown in formula 16 and 0.31mL of a n-hexane solution of n-butyl lithium with a molar concentration of 1.6mol / L were mixed in React at 0°C for 1 hour to obtain the first intermediate product; at 25°C, add 5 mL of the tetrahydrofuran solution of the first intermediate product with a concentration of 0.1 mol / L dropwise to 2 mL of YCl with a concentration of 0.25 mol / L 3 In the tetrahydrofuran suspension, react for 4 hours, obtain the second intermediate product; In the second intermediate product, add the LiCH of 1.0mmol 2 SiMe 3 After reacting for 12 hours, the obtained reaction product was desolventized, extracted with toluene and concentrated to obtain 0.24 g of the rare earth complex having the structure shown in Formula 1, and the yield of the rare earth complex having the structure shown in Formula 1 was 81%.
[0119]
[0120] Terminal gro...
Embodiment 2
[0122] According to the method described in Example 1, the rare earth complex with the structure shown in Formula 2 was prepared, and the difference from Example 1 was that the ligand with the structure shown in Formula 17 was used to replace the ligand with the structure shown in Formula 16 in Example 1. .
[0123] Example 2 of the present invention prepared 0.27 g of the rare earth complex having the structure shown in Formula 2, and the yield of the rare earth complex having the structure shown in Formula 2 was 84%.
[0124]
[0125] Terminal groups not given in Formula 17 are methyl groups.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com