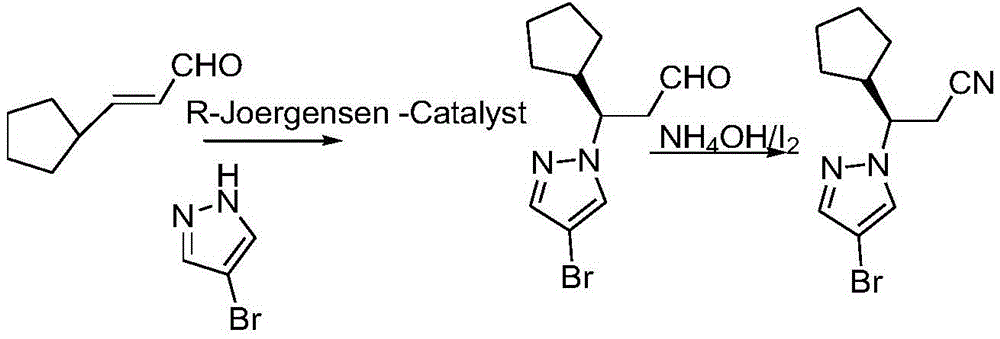
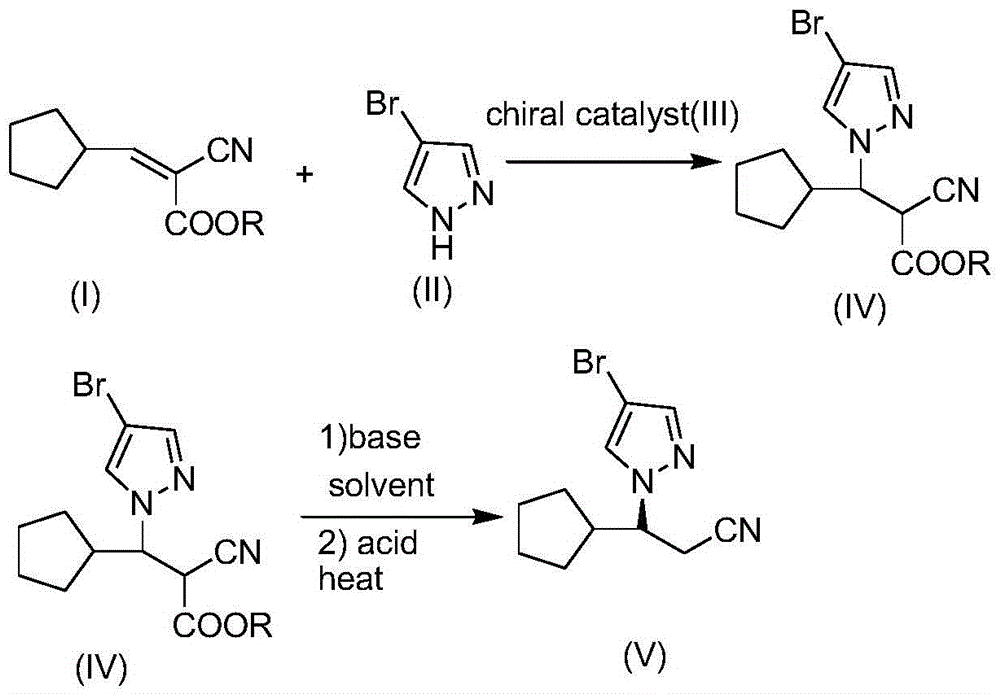
Method for synthesizing ruxolitinib intermediate (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentyl propionitrile
A technology of cyclopentylpropionitrile and a synthesis method, applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of harsh conditions, high catalyst cost, long route and the like, and achieves the effects of low cost, simple method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthetic method of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile is realized through the following steps:
[0037] 1) Preparation of ethyl (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentylpropionate
[0038] Add 1.93g (0.01mol) of ethyl 3-cyclopentyl-2-cyanoacrylate, 0.45g (0.001mol) of chiral square amide catalyst compound IIIa, and 1.59g of 4-bromo-1H-pyrazole into a 25ml single-necked bottle (0.0108mol), 15mL of dry toluene, react at -20°C for about 10 hours, follow the reaction by TLC, after the reaction is complete, concentrate under reduced pressure to remove the solvent, and the residue is directly used in the next reaction.
[0039] 2) Preparation of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile
[0040]Add 2.1g (0.013molNaOH) of 25% sodium hydroxide solution to the residue of step 1), heat up to 65°C and reflux for about 8 hours until TLC follows the reaction until the hydrolysis is complete, then use 35% hydrochloric acid A...
Embodiment 2
[0046] The synthetic method of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile is realized through the following steps:
[0047] 1) Preparation of (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentylpropionic acid methyl ester
[0048] Add 1.79g (0.01mol) of methyl 3-cyclopentyl-2-cyanoacrylate, 0.45g (0.001mol) of chiral square amide catalyst compound IIIa, and 1.61g of 4-bromo-1H-pyrazole into a 25ml single-necked bottle (0.011mol), 15mL of dry toluene, react at -50°C for about 10, follow the reaction by TLC, after the reaction is complete, concentrate under reduced pressure to remove the solvent, and the residue is directly used in the next reaction.
[0049] 2) Preparation of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile
[0050] Add 2.4g (0.015molNaOH) of sodium hydroxide solution with a mass fraction of 25% to the residue of step 1), heat up to 65°C and reflux for about 8 hours, follow the reaction by TLC until the hydrolysis is complete, and ad...
Embodiment 3
[0052] The synthetic method of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile is realized through the following steps:
[0053] 1) Preparation of (R)-3-(4-bromo-1H-pyrazol-1-yl)-2-cyano-3-cyclopentyl propionate propyl ester
[0054] Add 1.93g (0.01mol) of ethyl 3-cyclopentyl-2-cyanoacrylate, 0.45g (0.001mol) of chiral square amide catalyst compound IIIa, and 1.64g of 4-bromo-1H-pyrazole into a 25ml single-necked bottle (0.011mol), 15mL of dry toluene, reacted at 0°C, followed the reaction by TLC until the reaction was complete, concentrated under reduced pressure to remove the solvent, and the residue was directly used in the next reaction.
[0055] 2) Preparation of (R)-3-(4-bromo-1H-pyrazol-1-yl)-3-cyclopentylpropionitrile
[0056] Add 1.8 g (0.011 mol NaOH) of sodium hydroxide solution with a mass fraction of 25% to the residue, heat up to 65°C and reflux for about 8 hours, and follow the reaction by TLC until the hydrolysis is complete, adjust the pH to 2 with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com