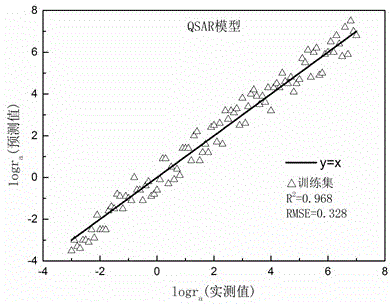
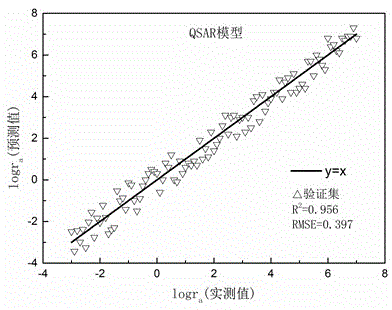
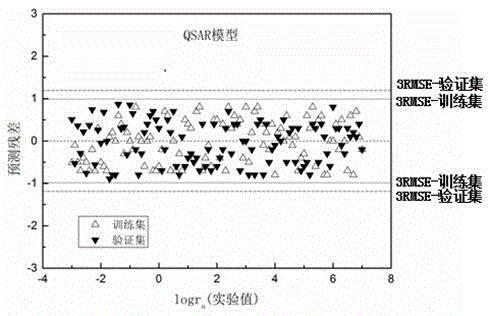
Method for predicting hydrolysis rate of sulfur-containing organic compounds in atmosphere
A technology of hydrolysis rate and organic matter, applied in special data processing applications, instruments, electrical digital data processing, etc., can solve problems that have not been reported before, and achieve the effects of wide application, simple calculation, and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment relates to a predictive CS 2 in Fe 2 o 3 Method for the rate of hydrolysis over a catalyst
[0028] (1) Under the condition of 50°C, use the quantum chemical software (MaterialsStudio) to analyze the CS 2 and H 2 The molecular structure of O was optimized by calculating the CS 2 with H 2 The energy difference of O before and after the reaction on the surface of the catalyst gives the hydrolysis equilibrium coefficient at 50 °C = 24.174. By calculating the adsorption energy of gas molecules and water molecules on the catalyst surface, the adsorption equilibrium coefficient = 21.885.
[0029] (2) and apply the QSAR model to predict the concentration of CS at 20ppm 2 Hydrolysis rate at 50 °C.
[0030] The QSAR model is:
[0031]
[0032] Among them, r a for CS 2 in Fe 2 o 3 Hydrolysis rate on catalyst (mol / s), Pa is CS 2 Partial pressure = 20 (ppm), P H2O Partial pressure of water vapor = 5000 (ppm), P c and P d respectively H 2 S and an...
Embodiment 2
[0041] This example relates to a method for predicting the hydrolysis rate of COS on a CuO catalyst
[0042] (1) Under the condition of 70°C, use quantum chemical software (MaterialsStudio) to analyze COS and H 2 The molecular structure of O was optimized by calculating COS and H 2 The energy difference of O before and after the reaction on the catalyst surface gives the hydrolysis equilibrium coefficient at 70 °C = 17.657. By calculating the adsorption energy of gas molecules and water molecules on the catalyst surface, the adsorption equilibrium coefficient = 15.809.
[0043] (2) And apply the QSAR model to predict the hydrolysis rate of COS at a concentration of 200ppm at 70°C.
[0044] The QSAR model is:
[0045]
[0046] Among them, r a is the hydrolysis rate of COS on CuO catalyst (mol / s), Pa is the partial pressure of COS = 200 (ppm), P H2O Partial pressure of water vapor = 10000 (ppm), P c and P d respectively H 2 S and another hydrolysis product CO 2 The part...
Embodiment 3
[0055] This embodiment relates to a method for predicting CH 3 SH in CeO 2 Method for the rate of hydrolysis over a catalyst
[0056] (1) Under the condition of 80°C, use quantum chemical software (MaterialsStudio) to analyze CH 3 SH and H 2 The molecular structure of O was optimized by calculating the CH 3 SH and H 2 The energy difference of O before and after the reaction on the catalyst surface gives the hydrolysis equilibrium coefficient at 80°C = 8.221. By calculating the adsorption energy of gas molecules and water molecules on the catalyst surface, the adsorption equilibrium coefficient = 6.256.
[0057] (2) and apply the QSAR model to predict CH at a concentration of 100ppm 3 Hydrolysis rate of SH at 80 °C.
[0058] The QSAR model is:
[0059]
[0060] Among them, r a for CH 3 SH in CeO 2 Hydrolysis rate on catalyst (mol / s), Pa is CH 3 Partial pressure of SH = 100 (ppm), P H2O Partial pressure of water vapor = 8000 (ppm), P c and P d respectively H 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com