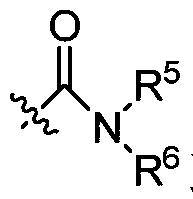
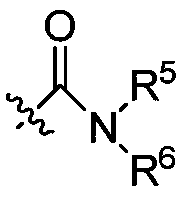
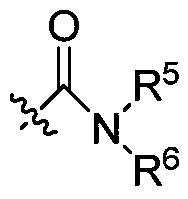
A nitrogen-containing condensed heterocyclic compound, its preparation method, composition and application
A compound and fused heterocycle technology, applied in the field of nitrogen-containing fused heterocycle compounds, can solve the problems of large clinical side effects, poor selectivity, low specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Embodiment 1' (preparation of intermediate):
[0142]
[0143] first step:
[0144] 2,6-dichloro-4-methylnicotine nitrile (5g, 26.7mmol) (compound shown in formula 1-a) was placed in a sealed tube, and tert-butylamine (3.12mL, 29.4mmol) and DIPEA were added thereto (5.3mL, 32.1mmol), heated to 120°C for 18h. After the reaction, the reaction solution was cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed with water, the organic phase was dried over anhydrous sodium sulfate, and purified by silica gel column chromatography (PE:EA=80:20 elution) to obtain 1.77 g of white solid (compound as shown in formula 1-b). 1 H-NMR (400MHz, CDCl 3 ):δ=6.15(1H,s),5.00(1H,s),2.37(3H,s),1.44(9H,s).LC-MS m / z:(M+H) + = 224.10.
[0145] Step two:
[0146] 6-(tert-butylamino)-2-chloro-4-methylnicotine nitrile (1.77g, 7.94mmol) (compound shown in formula 1-b) was dissolved in 40mL of glacial acetic acid, and the solution was added dropwise Bromi...
Embodiment 2
[0153] Embodiment 2' (preparation of intermediate):
[0154]
[0155] first step:
[0156] Dissolve sodium hydride (1.092g, 27.3mmol) in 25ml of anhydrous tetrahydrofuran, add diethyl cyanomethyl phosphate (2.66ml, 16.38mmol) to it under the protection of argon at 0°C, react at room temperature for 0.5h, and then add Add 1-(4-amino-2-(methylthio)pyrimidin-5-yl)ethanone (1g, 5.46mmol) (compound shown in formula I-16-e) dissolved in 5ml THF, room temperature React overnight. After the reaction was completed, water was slowly added to the reaction liquid to quench, and then extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, filtered, evaporated to dryness, purified by silica gel column chromatography (DCM:MeOH=97:3 washing off), to obtain 635 mg of yellow solid (compound shown in formula 2-a). 1 H-NMR (400MHz, d-DMSO): δ = 8.93 (1H, s), 7.33 (2H, s), 6.54 (1H, d, J = 1.2Hz), 2.51 (3H, s), 2.48 (3H, d,J=0.8Hz).LC-MS m / z:(M+H) + =207.00...
Embodiment 3
[0161] Embodiment 3' (preparation of intermediate):
[0162]
[0163] first step:
[0164] 1-(4-amino-2-(methylthio)pyrimidin-5-yl)ethanone (1g, 5.46mmol) (compound as shown in formula I-16-e) was dissolved in ethyl acetoacetate (3ml , 27.85mmol), heated to 180°C for 3h, after the reaction, cooled to room temperature, added 10ml of ethyl acetate to the reaction solution, filtered the insoluble solid, and dried to obtain 1.069g of yellow solid (as shown in formula 3-a compound of). 1 H-NMR (400MHz, d-DMSO): δ=12.60(1H,s), 9.02(1H,s), 2.58(3H,s), 2.44(3H,s), 2.34(3H,s).LC- MS m / z: (M+H) + =250.05.
[0165] Step two:
[0166] 6-acetyl-5-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin-7(8H)-one (0.353g, 1.42mmol) (as shown in formula 3-a The compound) was dissolved in phosphorus oxychloride (3ml, 32.77mmol), heated to 110°C to react overnight, after the reaction was completed, cooled to room temperature, evaporated to dryness, the residue was dissolved in saturated sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com