Production process of bovine trypsin
A bovine trypsin and production process technology, applied in the direction of enzyme, peptidase, hydrolase, etc., can solve the problems of poor effect and low yield, achieve high expression, low process cost, renaturation and subsequent purification steps simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The production technology of embodiment 1 bovine trypsin
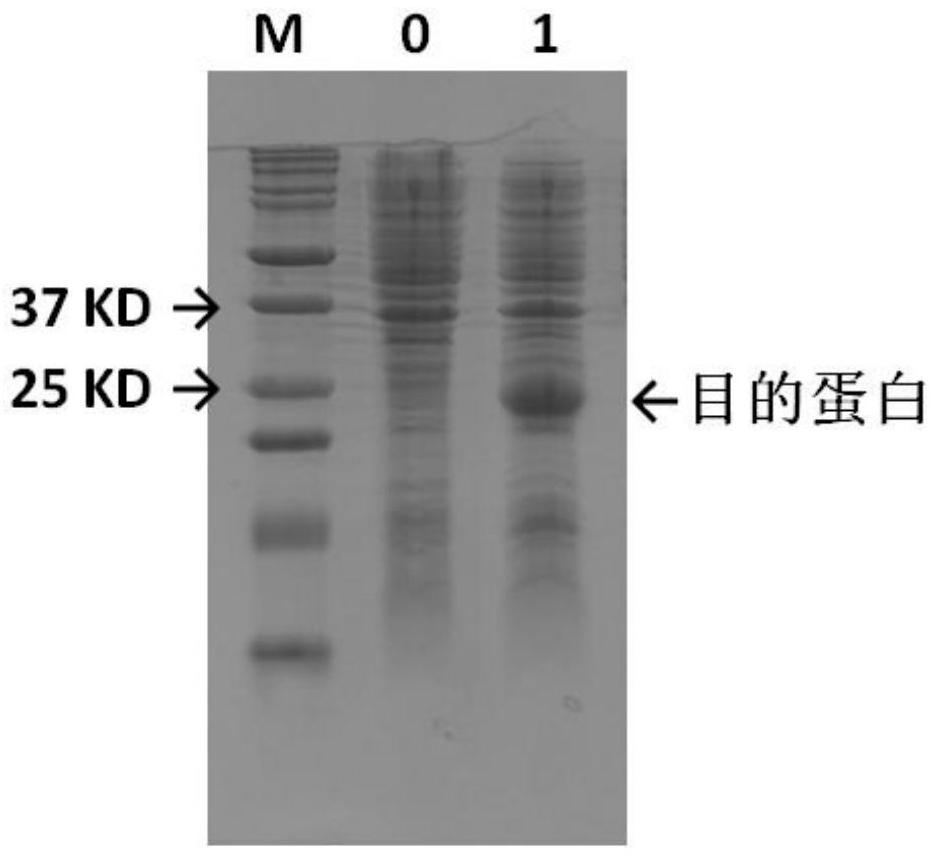
[0033] 1. Construction of prokaryotic production strains
[0034]The amino acid sequence of bTrypsin (bovine trypsinogen) is shown in SEQ ID NO:1. The nucleic acid sequence of bovine trypsinogen is deduced according to its amino acid sequence, and the codon is optimized to make it suitable for expression in genetically engineered bacteria. The nucleic acid sequence is shown in SEQ ID NO:2.
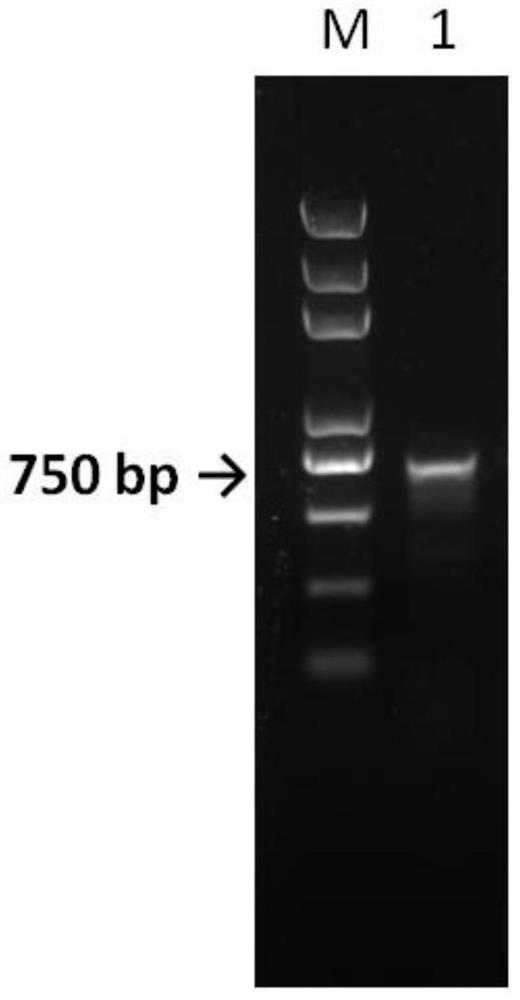
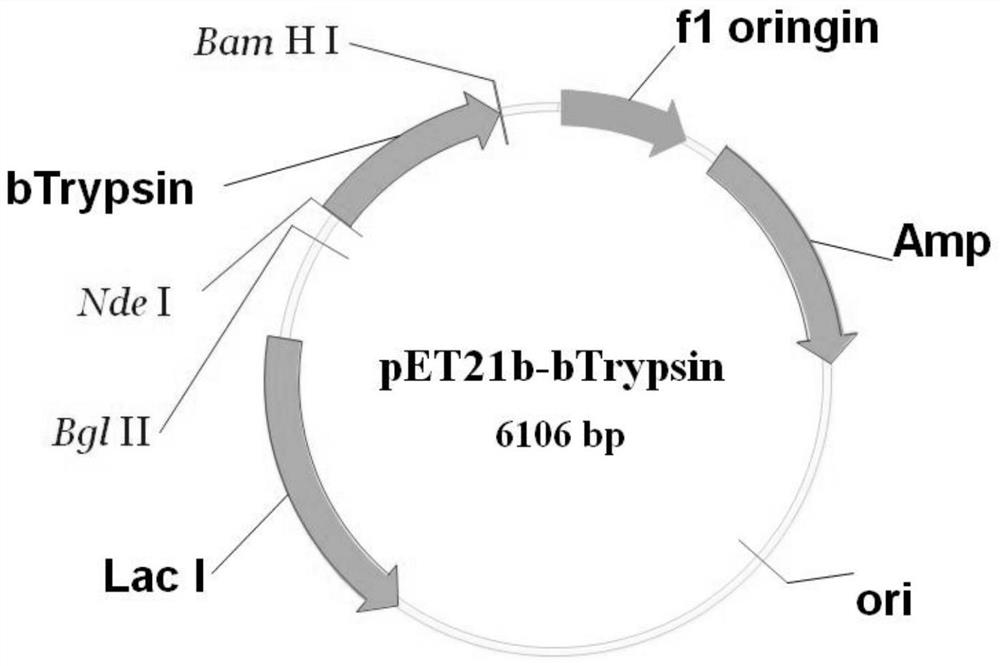
[0035] Using the method of whole gene synthesis, the gene is synthesized. bTrypsin was cloned into the commercial expression vector pET21b by means of restriction endonucleases NdeI and BamHl. The experimental procedure is as follows:
[0036] PCR technology was used to amplify the target fragment from the cloning vector.
[0037] The primer sequences are as follows (5'-3'):
[0038] bTrypsin21b-F:GGGAATTCCATATGATCGTTGGTGGTTACACCTG
[0039] bTrypsin21b-R:CGCGGATCCTTAGTTAGAAGCGATGGTCTGT
[0040] The PCR reaction syste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com