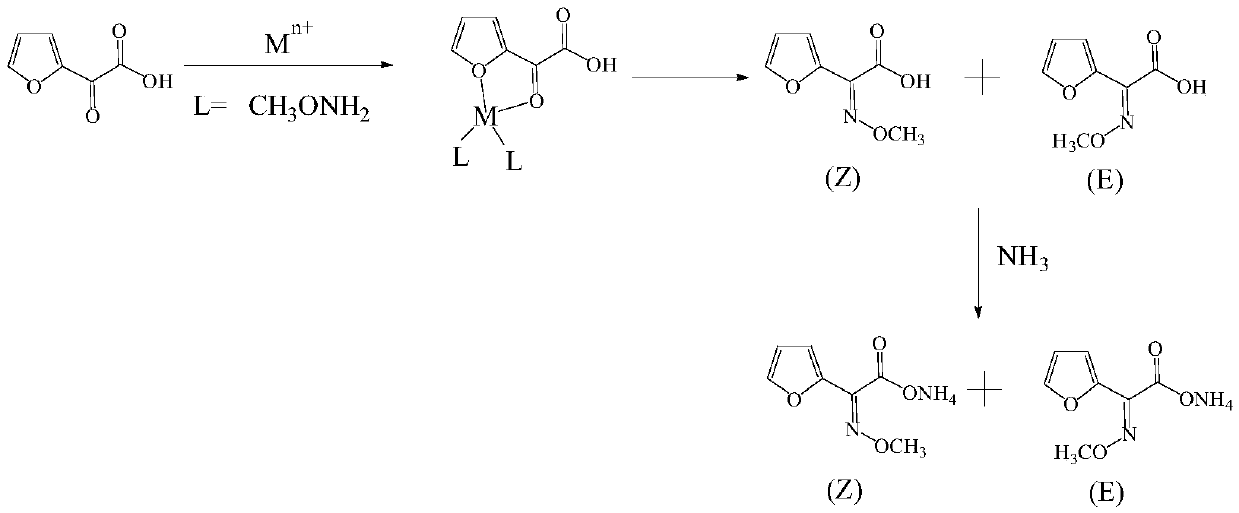
Method for synthesizing (Z)-2-(alpha-methoxyimino)furanylacetic acid ammonium
A technology of ammonium furanacetate and methoxyimine, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of difficult product refinement, unfavorable production, increased cost, etc., achieve high yield, good product quality, and reduce resources wasteful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 40g 2-oxo-2-furyl acetic acid and 0.04g copper sulfate pentahydrate in 300mL water, add 100g methoxyamine aqueous solution at 0°C, adjust the pH to 3.5 with dilute sulfuric acid;
[0027] The mass fraction of the methoxyamine aqueous solution is 17%;
[0028] (2) Incubate the solution prepared in step (1) at 8°C for 4.5 hours to obtain 2-(α-methoxyimine) furan acetic acid solution; liquid phase detection, (Z)-2-(α-methoxy Imine) furan acetic acid: (E)-2-(α-methoxyimine) furan acetic acid is 95.1:4.9;
[0029] (3) Adjust the pH of the 2-(α-methoxyimine) furan acetic acid solution to 0.8 with dilute sulfuric acid, control the temperature at 20°C, extract with dichloromethane, and combine the organic phases;
[0030] (4) Ammonia gas was passed into the organic phase at 5°C, the pH was adjusted to 7.0, and the crude product was obtained after heat preservation for 1 hour. After decolorization, concentration and crystallization, 48.9 g of the product was obtained, and t...
Embodiment 2
[0038] (1) Dissolve 40g 2-oxo-2-furylacetic acid and 0.032g manganese dichloride in 300mL water, add 160g methoxyamine hydrochloride aqueous solution at 5°C, and adjust the pH to 3.0 with potassium hydroxide;
[0039] The mass fraction of the methoxyamine hydrochloride aqueous solution is 20%;
[0040] (2) Incubate the solution prepared in step (1) at 10°C for 2 hours to obtain a 2-(α-methoxyimine) furan acetic acid solution; liquid phase detection, (Z)-2-(α-methoxy Amine) furan acetic acid: (E)-2-(α-methoxyimine) furan acetic acid is 95.0:5.0;
[0041] (3) Adjust the pH of the 2-(α-methoxyimine) furan acetic acid solution to 0.1 with dilute hydrochloric acid, control the temperature at 25°C, extract with dichloromethane, and combine the organic phases;
[0042] (4) Ammonia gas was passed into the organic phase at 0°C, the pH was adjusted to 7.5, and the crude product was obtained after heat preservation for 1.5 hours. After decolorization, concentration and crystallization, 47.4 g of...
Embodiment 3
[0050] (1) Dissolve 40g 2-oxo-2-furyl acetic acid and 0.048g zinc sulfate in 300mL water, add 392g methoxyamine aqueous solution at 10°C, adjust the pH to 2.5 with dilute sulfuric acid;
[0051] The mass fraction of the methoxyamine aqueous solution is 5%;
[0052] (2) Incubate the solution prepared in step (1) at 5°C for 7 hours to obtain a 2-(α-methoxyimine) furan acetic acid solution; liquid phase detection (Z)-2-(α-methoxyimine) ) Furan acetic acid: (E)-2-(α-methoxyimine) furan acetic acid is 95.5:4.5,
[0053] (3) Adjust the pH of the 2-(α-methoxyimine) furan acetic acid solution to 1.5 with dilute phosphoric acid, control the temperature at 15°C, extract with dichloromethane, and combine the organic phases;
[0054] (4) Pass liquid ammonia into the organic phase at 10°C, adjust the pH to 6.5, and keep the crude product for 0.5h. After decolorization, concentration and crystallization, the product is 48.0g, and the yield is 90%.
[0055] Product test results:
[0056] Appearance: o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Moisture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com