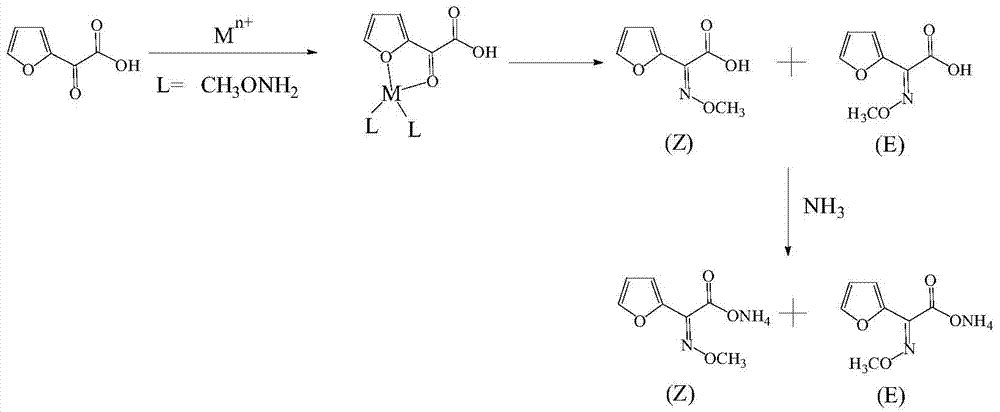
Method for synthesizing (z)‑2‑(α‑methoxyimine) ammonium furoacetate
A technology of ammonium furanacetate and methoxyimine, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of difficult product refinement, unfavorable production, increased cost, etc., achieve high yield, good product quality, and reduce resources wasteful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 40g of 2-oxo-2-furyl acetic acid and 0.04g of copper sulfate pentahydrate in 300mL of water, add 100g of methoxyamine aqueous solution at 0°C, and adjust the pH to 3.5 with dilute sulfuric acid;
[0027] The massfraction of described methoxyamine aqueous solution is 17%;
[0028] (2) Insulate the solution prepared in step (1) at 8°C for 4.5h to obtain 2-(α-methoxyimine)furan acetic acid solution; liquid phase detection, (Z)-2-(α-methoxy Imine)furanacetic acid: (E)-2-(α-methoxyimine)furanacetic acid is 95.1:4.9;
[0029] (3) Use dilute sulfuric acid to adjust the pH of the 2-(α-methoxyimino)furan acetic acid solution to 0.8, control the temperature to 20°C, extract with dichloromethane, and combine the organic phases;
[0030] (4) Ammonia gas was introduced into the organic phase at 5°C, the pH was adjusted to 7.0, and the crude product was obtained after heat preservation for 1 hour. After decolorization, concentration and crystallization, 48.9 g of the pr...
Embodiment 2
[0038] (1) Dissolve 40g of 2-oxo-2-furylacetic acid and 0.032g of manganese dichloride in 300mL of water, add 160g of methoxyamine hydrochloride aqueous solution at 5°C, and adjust the pH to 3.0 with potassium hydroxide;
[0039] The massfraction of described methoxyamine hydrochloride aqueous solution is 20%;
[0040] (2) Insulate the solution prepared in step (1) at 10°C for 2 hours to obtain 2-(α-methoxyimine)furan acetic acid solution; liquid phase detection, (Z)-2-(α-methoxyimine) Amine) furan acetic acid: (E)-2-(α-methoxyimine) furan acetic acid is 95.0:5.0;
[0041] (3) Use dilute hydrochloric acid to adjust the pH of the 2-(α-methoxyimino)furan acetic acid solution to 0.1, control the temperature to 25° C., extract with dichloromethane, and combine the organic phases;
[0042] (4) Ammonia gas was introduced into the organic phase at 0°C, the pH was adjusted to 7.5, and the crude product was obtained after heat preservation for 1.5 h. After decolorization, concentratio...
Embodiment 3
[0050] (1) Dissolve 40g of 2-oxo-2-furyl acetic acid and 0.048g of zinc sulfate in 300mL of water, add 392g of methoxyamine aqueous solution at 10°C, and adjust the pH to 2.5 with dilute sulfuric acid;
[0051] The massfraction of described methoxyamine aqueous solution is 5%;
[0052] (2) The solution prepared in step (1) was incubated at 5° C. for 7 hours to obtain a 2-(α-methoxyimine) furan acetic acid solution; liquid phase detection (Z)-2-(α-methoxyimine ) furan acetic acid: (E)-2-(α-methoxyimine) furan acetic acid is 95.5:4.5,
[0053] (3) Use dilute phosphoric acid to adjust the pH of the 2-(α-methoxyimino)furan acetic acid solution to 1.5, control the temperature to 15°C, extract with dichloromethane, and combine the organic phases;
[0054] (4) Pass liquid ammonia into the organic phase at 10° C., adjust the pH to 6.5, and keep warm for 0.5 h to obtain the crude product. After decolorization, concentration and crystallization, 48.0 g of the product is obtained with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com