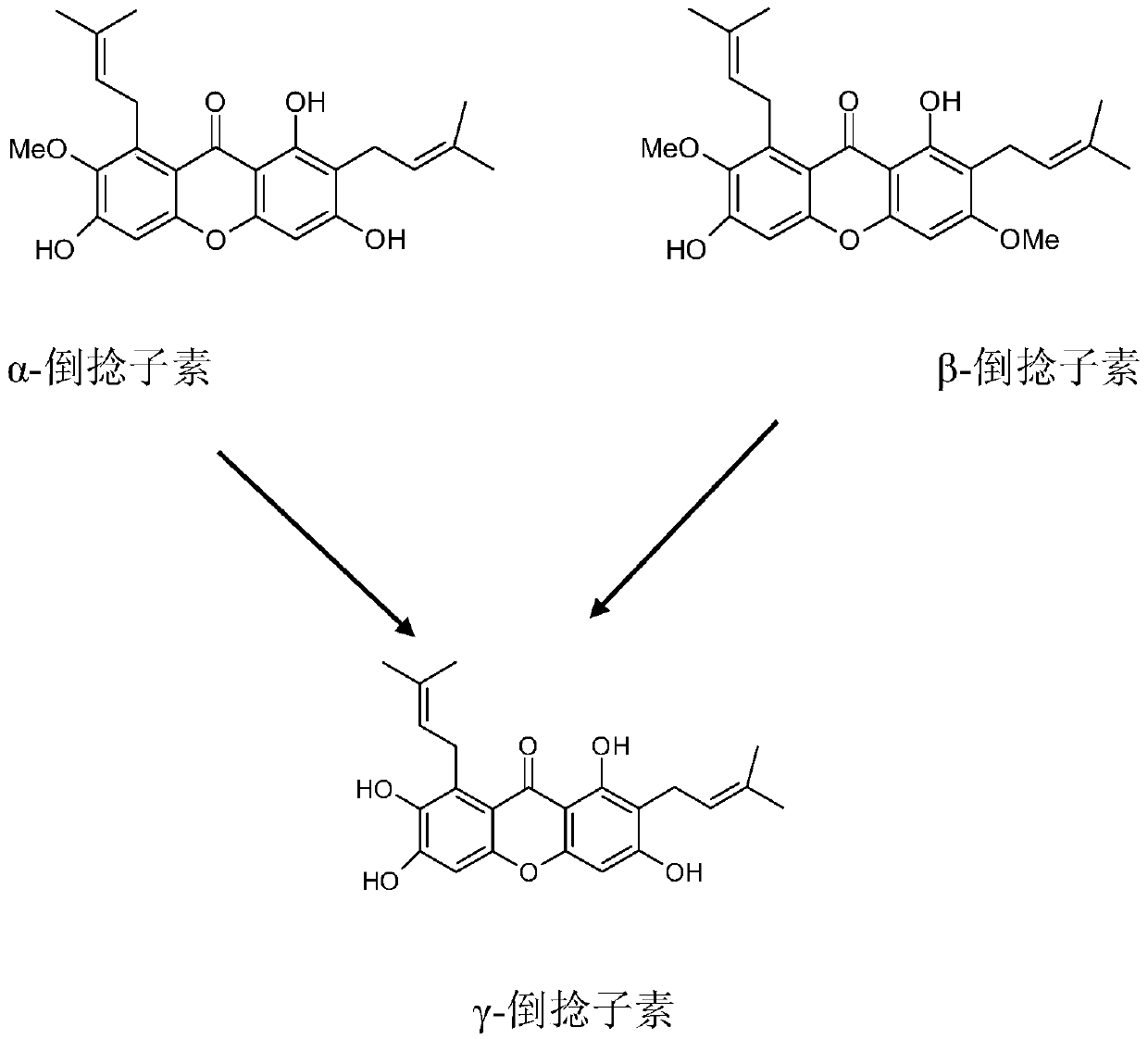
Synthetic method of gamma-mangostin
A technology of mangostin and synthetic method, which is applied in the biological field to achieve the effect of high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1. Add 40 g of mangosteen extract, 300 ml of dichloroethane, 300 ml of pyridine, 64 g of anhydrous aluminum trichloride, and 0.1 g of sodium iodide into a 1000 ml reaction flask. Heat to reflux for three hours. After cooling the reaction solution, add 6g of concentrated hydrochloric acid to neutralize it until the reaction solution is weakly acidic, reclaim dichloroethane under reduced pressure, place and cool to obtain 30g of crude product of gamma-mangostin.
[0027] Step 2. Recrystallize 30 g of the γ-mangostin crude product obtained in the above steps with 600 ml of absolute ethanol, add 5 g of activated carbon for decolorization, concentrate the ethanol to one-third of the original volume, place it for crystallization, and suction filter after 24 hours to obtain 98 % gamma-mangostin essence 21g.
Embodiment 2
[0029] Step 1. Add 40 g of mangosteen extract, 300 ml of dichloroethane, 150 ml of pyridine, 64 g of anhydrous aluminum trichloride, and 0.1 g of sodium iodide into a 1000 ml reaction flask. Heat to reflux for three hours. After cooling the reaction solution, add 4g concentrated hydrochloric acid to neutralize it until the reaction solution is weakly acidic, reclaim dichloroethane under reduced pressure, place and cool to obtain 29g of γ-mangostin crude product.
[0030] Step 2. Recrystallize 29 g of the γ-mangostin crude product obtained in the above steps with 600 ml of absolute ethanol, add 6 g of activated carbon for decolorization, concentrate the ethanol to one-third of the original volume, place it for crystallization, and suction filter after 24 hours to obtain 98 % gamma-mangostin essence 22g.
Embodiment 3
[0032] Step 1. Add 40 g of mangosteen extract, 300 ml of dichloroethane, 150 ml of triethylamine, 64 g of anhydrous aluminum trichloride, and 0.2 g of sodium iodide into a 1000 ml reaction flask. Heat to reflux for three hours. After cooling the reaction solution, add 4g of concentrated hydrochloric acid to neutralize it until the reaction solution is weakly acidic, reclaim dichloroethane under reduced pressure, place and cool to obtain 28g of γ-mangostin crude product.
[0033] Step 2. Recrystallize 28 g of the γ-mangostin crude product obtained in the above steps with 560 ml of absolute ethanol, add 4 g of activated carbon for decolorization, concentrate the ethanol to one-third of the original volume, place it for crystallization, and suction filter after 24 hours to obtain 98 % gamma-mangostin essence 20g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com