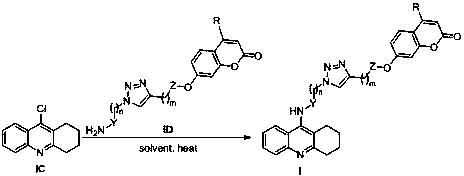
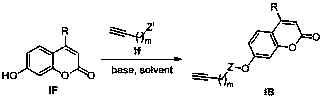
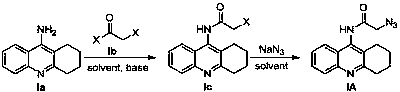
Tacrine-coumarin derivative containing triazole and application of derivative
A technology of coumarin derivatives and triazole bases, applied in the field of biomedicine, can solve the problems of low bioavailability, high liver toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Synthesis of 2-(7-(2-carbonyl-4-methyl-2H-chromene)methoxy)propargyl acetate
[0066] Dissolve propargyl alcohol (10mmol) in dry dichloromethane (20mL), add triethylamine (11mmol), cool in an ice bath to 0°C, add chloroacetyl chloride (11mmol) dropwise, the reaction solution gradually turns brown, and the dropwise After that, the temperature was raised naturally for 2 hours, the reaction was stopped, washed with water, extracted with dichloromethane, the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a reddish-brown liquid, which was dissolved in acetone / tetrahydrofuran and mixed Solvent (15mL, v:v=1:1), add 7-hydroxycoumarin (7mmol), potassium carbonate (7mmol), heat to 60°C for about 12h, stop the reaction, wash with water, extract with dichloromethane, organic After the phases were combined, they were washed once with saturated brine, dried over anhydrous sodium sulfate, filtere...
Embodiment 2
[0069] Synthesis of 2-(7-(2-carbonyl-4-methyl-2H-chromene)methoxy)propargyl acetate
[0070] Dissolve propargyl alcohol (10mmol) in dry dichloromethane (20mL), add triethylamine (11mmol), cool in an ice bath to 0°C, add chloroacetyl chloride (11mmol) dropwise, the reaction solution gradually turns brown, and the dropwise After that, the temperature was raised naturally for 2 hours, the reaction was stopped, washed with water, extracted with dichloromethane, the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a reddish-brown liquid, which was dissolved in acetone / tetrahydrofuran and mixed Solvent (15mL, v:v=1:1), add 4-methyl-7-hydroxycoumarin (7mmol), potassium carbonate (7mmol), heat to 60°C for about 12h, stop the reaction, add water to wash, two Extracted with methyl chloride, combined the organic phases and washed once with saturated brine, dried over anhydrous sodium sulfate, filtered...
Embodiment 3
[0073] Synthesis of 2-(7-(2-carbonyl-4-phenyl-2H-chromene)methoxy)propargyl acetate
[0074] Dissolve propargyl alcohol (10mmol) in dry dichloromethane (20mL), add triethylamine (11mmol), cool in an ice bath to 0°C, add chloroacetyl chloride (11mmol) dropwise, the reaction solution gradually turns brown, and the dropwise After that, the temperature was raised naturally for 2 hours, the reaction was stopped, washed with water, extracted with dichloromethane, the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a reddish-brown liquid, which was dissolved in acetone / tetrahydrofuran and mixed Solvent (15mL, v:v=1:1), add 4-phenyl-7-hydroxycoumarin (7mmol), potassium carbonate (7mmol), heat to 60°C for about 12h, stop the reaction, add water to wash, two After extraction with methyl chloride, the organic phases were combined and washed once with saturated brine, dried over anhydrous sodium sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com