Porcine parvovirus-porcine pseudorabies combined inactivated vaccine, and preparation method and application thereof
A dual inactivated vaccine, porcine pseudorabies technology, applied in the directions of biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of complex immunization procedures and high costs, and achieve optimized immunization procedures, good safety, and good safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
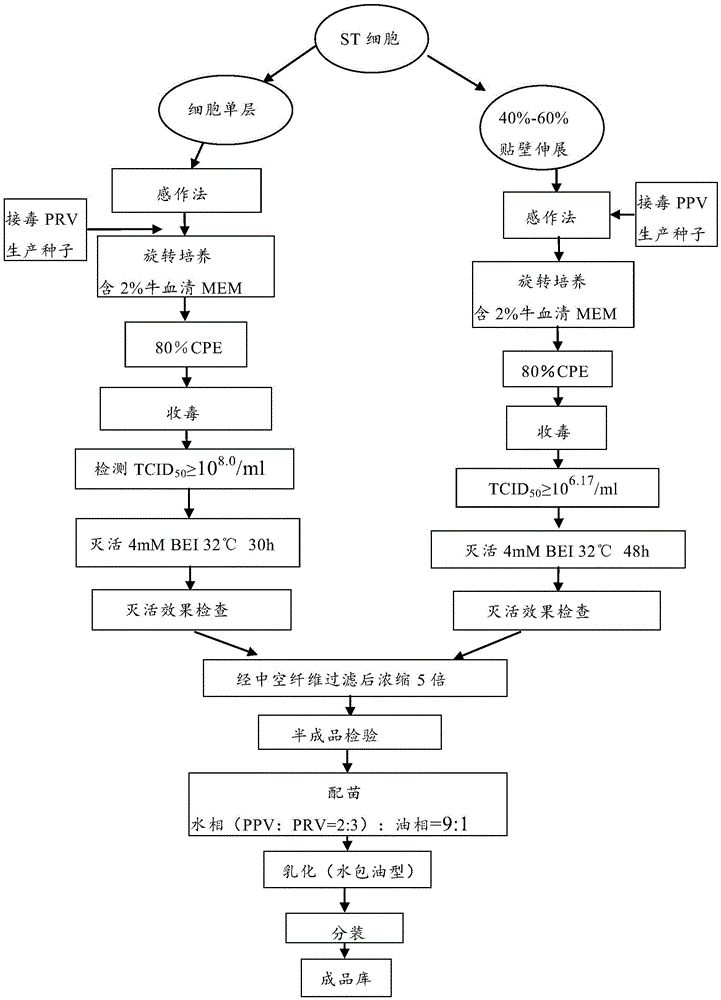
[0032] Preparation and inspection of the dual inactivated vaccine of porcine parvovirus disease-porcine pseudorabies of embodiment 1
[0033] 1.1 Preparation of poisonous seeds for production
[0034] Preparation of PPVL strain: Dilute the virus seed appropriately with the virus diluent (serum-free MEM medium), inoculate it into a single-layer ST cell culture at a multiplicity of infection (M.O.I.) of 0.01, and place it in a 37°C rotary culture. When the lesion reaches 80%, harvest the toxic cell culture medium, freeze and thaw 3 times, store at -20°C, and the virus titer should be ≥10 6.17 TCID 50 / ml.
[0035] Preparation of PRV-JL strain: Dilute the virus seeds appropriately with virus diluent (serum-free MEM medium), and inoculate them in ST cell cultures that form a monolayer of 40% to 60% according to the multiplicity of infection (M.O.I.) of 0.01 , placed at 37°C for rotary culture, when the lesion reached 80%, harvested the toxic cell culture medium, frozen and thaw...
Embodiment 2
[0096] The dual inactivated vaccine prepared by the different proportions of the vaccine of embodiment 2PPV, PRV is compared with each single vaccine (PPV inactivated vaccine and PRV inactivated vaccine) immune effect
[0097] 1. Vaccines
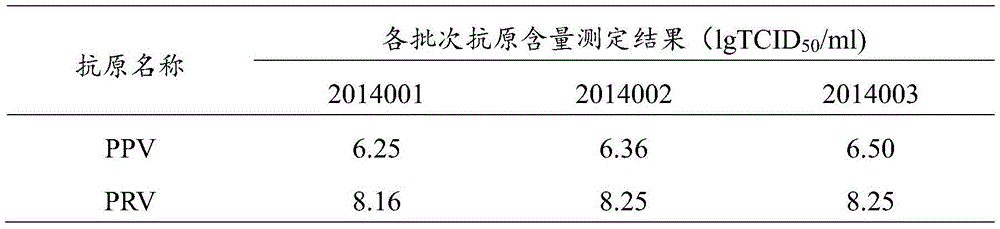
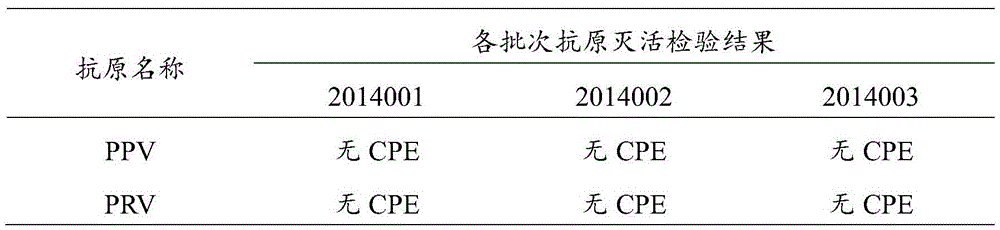
[0098] PPV, PRV double inactivated vaccine, select the laboratory product among the embodiment 1 (batch number is 2014001, PPV: PRV=2:3) and according to the method for embodiment 1, porcine parvovirus antigen, porcine pseudorabies virus antigen are respectively PPV by volume ratio: PRV=5:1, 4:1, 3:1, 5:2, 2:1, 3:2, 1:1, 3:4, 1:2, 2:5, 1:3 , 1:4, 1:5 prepared double vaccine; PPV disease inactivated vaccine single vaccine (batch number 201406); PRV inactivated vaccine single vaccine (batch number 201407).
[0099] 2. Design of animal experiments
[0100] 2.1 Parvovirus fraction
[0101] Adult guinea pigs with a body weight of 350 g or more and an HI antibody titer ≤ 1:8 were randomly divided into 16 groups, with 20 guinea pigs in each gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com