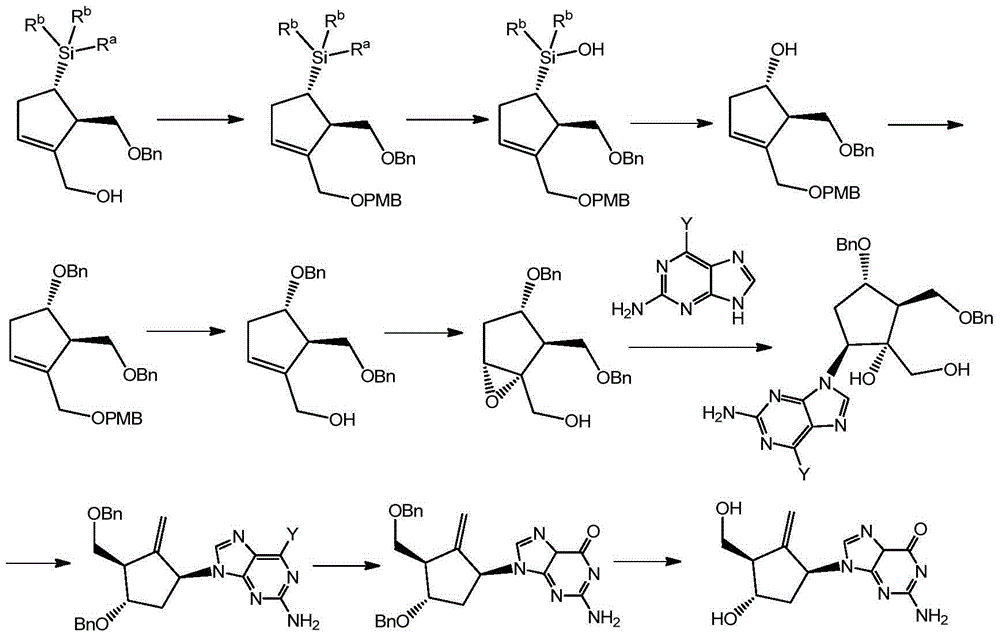
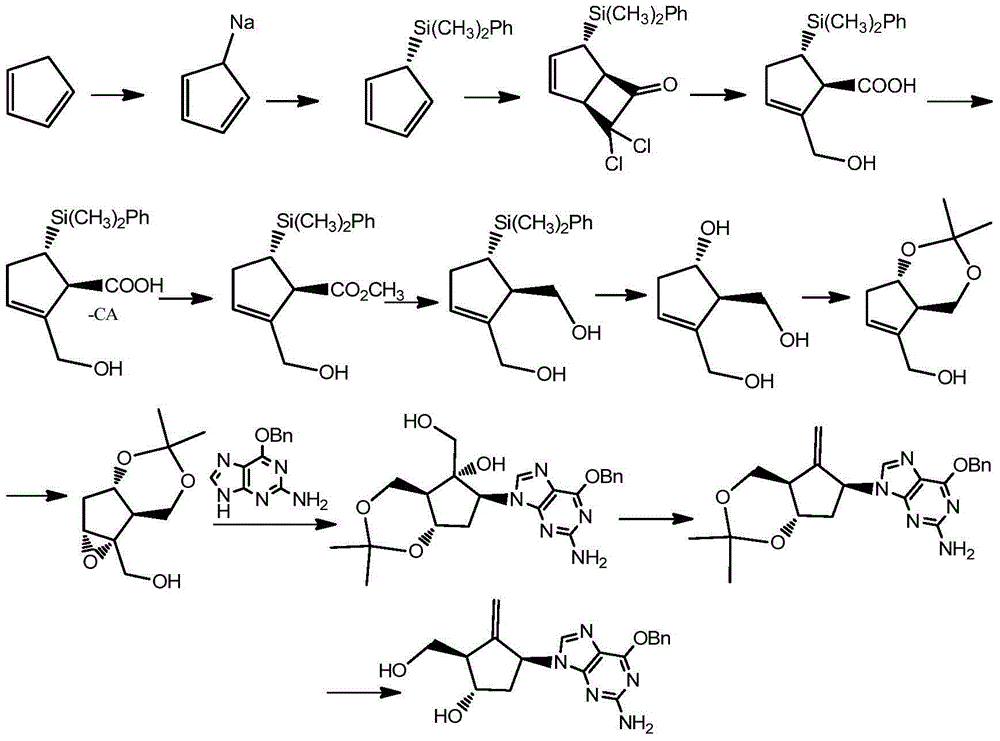
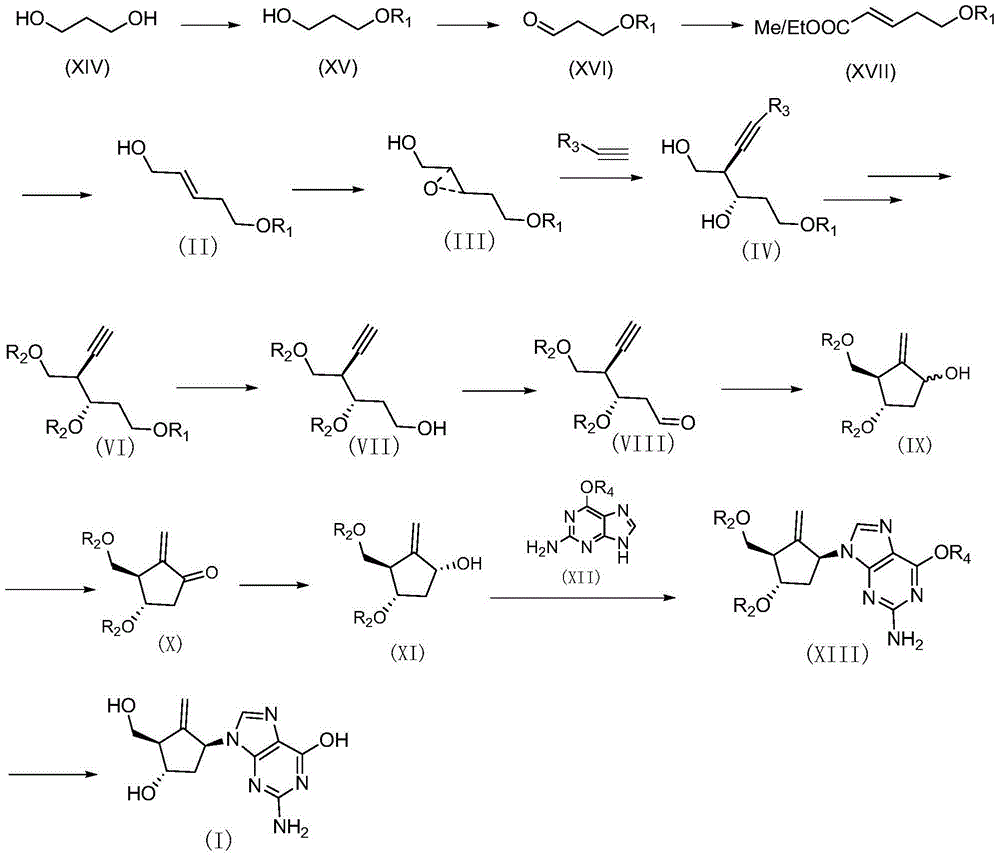
Method for synthesizing Entecavir
A technology of entecavir and synthetic methods, which is applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, high cost of process routes, and rare raw materials, and achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A synthetic method for entecavir, comprising the following steps:
[0059] (1) Preparation of compound XV
[0060] Add 1,3-propanediol (1457.6g, 19.15mol), DMF (dimethylformamide) (5L), DCM (dichloromethane) (1L) in a 20L reactor with temperature control and mechanical stirring, and then When the system is lower than 10°C under mechanical stirring, slowly add 60% NaH (281g, 7.024mol) into the kettle for about 2 hours, control the temperature in the reaction kettle not higher than 15°C, and keep the temperature in the kettle below 70min. Stirring was continued at 10°C for 30min.; then TBAI (tetrabutylammonium iodide) (23.6g, 0.064mol) was added, and the addition was completed in 15min; PMBCl (p-methoxybenzyl) (1000g, 6.385 mol), slowly dropwise into the kettle, 4h. The dropwise addition was completed; then the system was raised to room temperature and stirred overnight. TLC showed that the raw material was completely consumed. Then, lower the temperature of the system...
Embodiment 3
[0098] The preparation method of the present embodiment is basically the same as the preparation method in Example 1, the difference is:
[0099] In step (10), the preparation method of compound IX is as follows:
[0100] Under argon protection, add SmI to a 2000ml two-necked bottle 2 (Samarium diiodide) (812ml, 0.1M tetrahydrofuran solution, 81.2mmol) was placed in a low-temperature tank at -40°C and stirred; in another 25ml two-necked bottle with argon protection, the compound (VIII) obtained in the previous step was added ( 7.5g, 20.3mmol), anhydrous tert-butanol (4.5g, 60.8mmol), anhydrous THF (50ml), placed in a low-temperature tank at -40°C and stirred, and after the system remained stable at -40°C, drop it Add it to the reaction system at -40°C above, and drop it in 5 minutes. The reaction was tracked by TLC, and the raw material was consumed within 15 min. Add saturated aqueous ammonium chloride solution to quench the reaction, and add EA to it, let stand to separat...
Embodiment 4
[0102] The preparation method of the present embodiment is basically the same as the preparation method in Example 1, the difference is:
[0103] In step (10), the preparation method of compound IX is as follows:
[0104] Under the protection of argon, metal sodium (1.55g, 67.45mmol) was added to 100ml of liquid ammonia under stirring at -50°C, compound VIII (5.00g, 13.49mmol) was diluted with 50ml of anhydrous THF and added to the reaction system, and stirred 15min.; then place it at room temperature until all the liquid ammonia has evaporated. Add 50ml of saturated ammonium chloride aqueous solution to quench the reaction, add 100ml of EA, wash with saturated brine (3×50ml), anhydrous Na 2 SO 4 Dry, filter, concentrate, separate and purify to obtain 3.08g yellow liquid as compound (IX). Yield: 61.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com