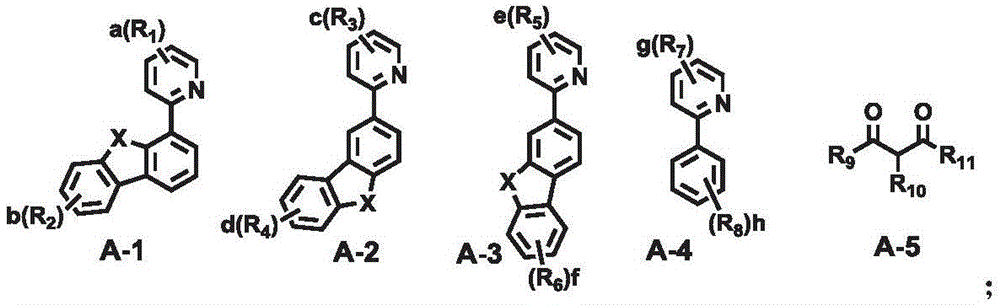
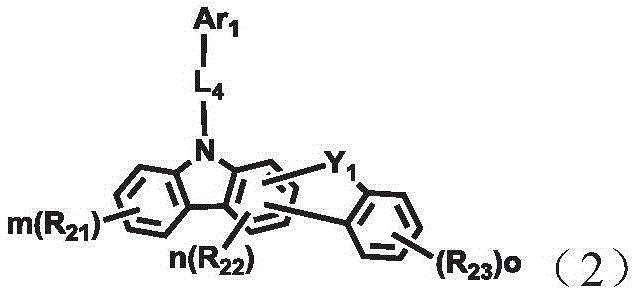
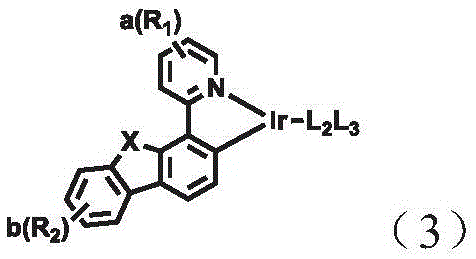A combination of a host compound and a dopant compound
A compound and dopant technology, which is applied in the direction of indium organic compounds, platinum group organic compounds, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of unmentioned combinations, low glass transition temperature, and power efficiency. Advantages and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0074] Example 1: Preparation of Compound D-2
[0075]
[0076] Preparation of compound 1-1
[0077] 2-Phenylpyridine (10.0g, 32.0mmol), iridium(III) chloride hydrate (IrCl 3 ·xH 2 O) (8.1 g, 29.0 mmol), 2-ethoxyethanol (220.0 mL) and H 2 O (74.0 mL) was stirred in the flask at 140 °C for 24 hours. After the reaction was complete, the mixture was cooled to room temperature and washed with H 2 Washed with O and methanol (MeOH), and then dried to obtain compound 1-1 (11.0 g, 71%).
[0078] Preparation of compound 1-2
[0079] Compound 1-1 (10.0 g, 9.0 mmol) was dissolved in dichloromethane (MC) (4.0 L) in a flask. Silver triflate (AgOTf) (5.0 g, 19.0 mmol) dissolved in MeOH (400.0 mL) was slowly added thereto and the mixture was stirred at room temperature for 12 hours. After the reaction was completed, the reaction mixture was filtered and the filtrate was dried to obtain compound 1-2 (12.0 g, 94%).
[0080] Preparation of Compound 1-3
[0081] 2-Bromopyridin...
example 2
[0085] Example 2: Preparation of Compound D-3
[0086]
[0087] Preparation of compound 2-1
[0088] 2-Bromo-5-methylpyridine (15.0g, 87.0mmol), phenylboronic acid (14.0g, 114.0mmol), Pd(PPh 3 ) 4 (3.0g, 2.6mmol), K 2 CO 3 (36.0g, 260.0mmol), toluene (300.0mL), EtOH (150.0mL) and H 2 O (130.0 mL) was stirred in the flask at 100 °C for 3 hours. After the reaction was complete, the mixture was extracted with EA, by using MgSO 4 The remaining water was removed and the mixture was distilled under reduced pressure. By column chromatography with MC / Hx=1 / 2, compound 2-1 (10.0 g, 68%) was obtained as a white solid.
[0089] Preparation of Compound 2-2
[0090] Compound 2-1 (10.0g, 30.0mmol), IrCl 3 ·xH 2 O (8.0 g, 27.0 mmol), 2-ethoxyethanol (200.0 mL) and H 2 O (70.0 mL) was stirred in the flask at 140 °C for 24 hours. After the reaction was complete, the mixture was cooled to room temperature and washed with H 2 Washed with O and MeOH, and then dried to obtain ...
example 3
[0096] Example 3: Preparation of Compound D-95
[0097]
[0098] Preparation of compound 3-1
[0099] 2-Bromo-4-methylpyridine (10.0g, 63.0mmol), dibenzo[b,d]furan-4-ylboronic acid (15.0g, 76.0mmol), Pd(PPh 3 ) 4 (2.2g, 2.0mmol), Na 2 CO 3 (20.0g, 19.0mmol), toluene (300.0mL), EtOH (150.0mL) and H 2 O (10.0 mL) was stirred in the flask at 100 °C for 2 hours. After the reaction was complete, the mixture was cooled to room temperature and extracted with EA. By using MgSO 4 The remaining water was removed and the mixture was distilled under reduced pressure. By column chromatography with MC / Hx=1 / 3, Compound 3-1 (11.0 g, 67%) was obtained as a white solid.
[0100] Preparation of Compound D-95
[0101] MeOH (200.0 mL) and EtOH (100.0 mL) were added to compound 3-1 (7.0 g, 28.0 mmol) and compound 2-3 (10.0 g, 14.0 mmol) in a flask and the mixture was stirred at reflux for 12 hours. After the reaction was complete, the mixture was cooled to room temperature and fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com